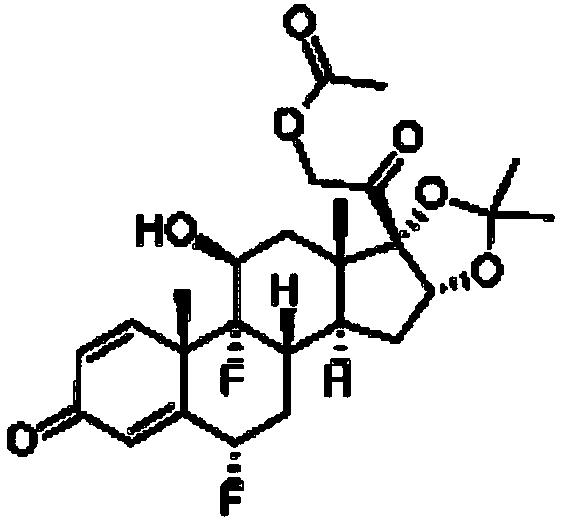
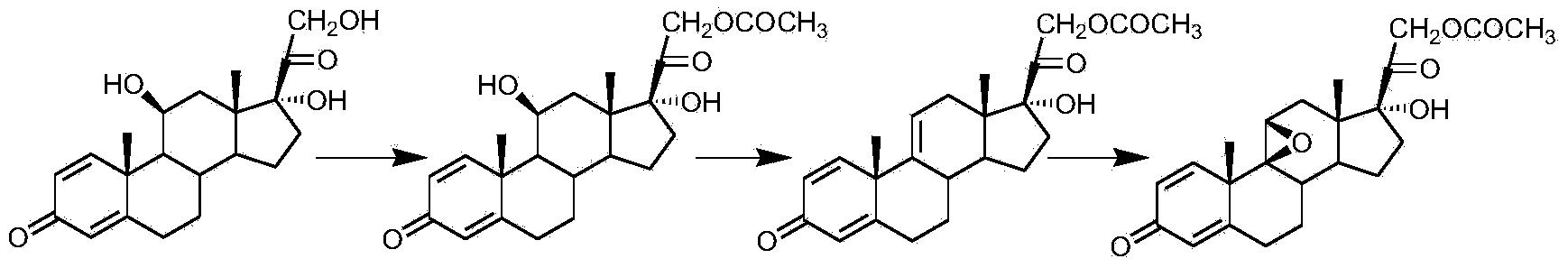
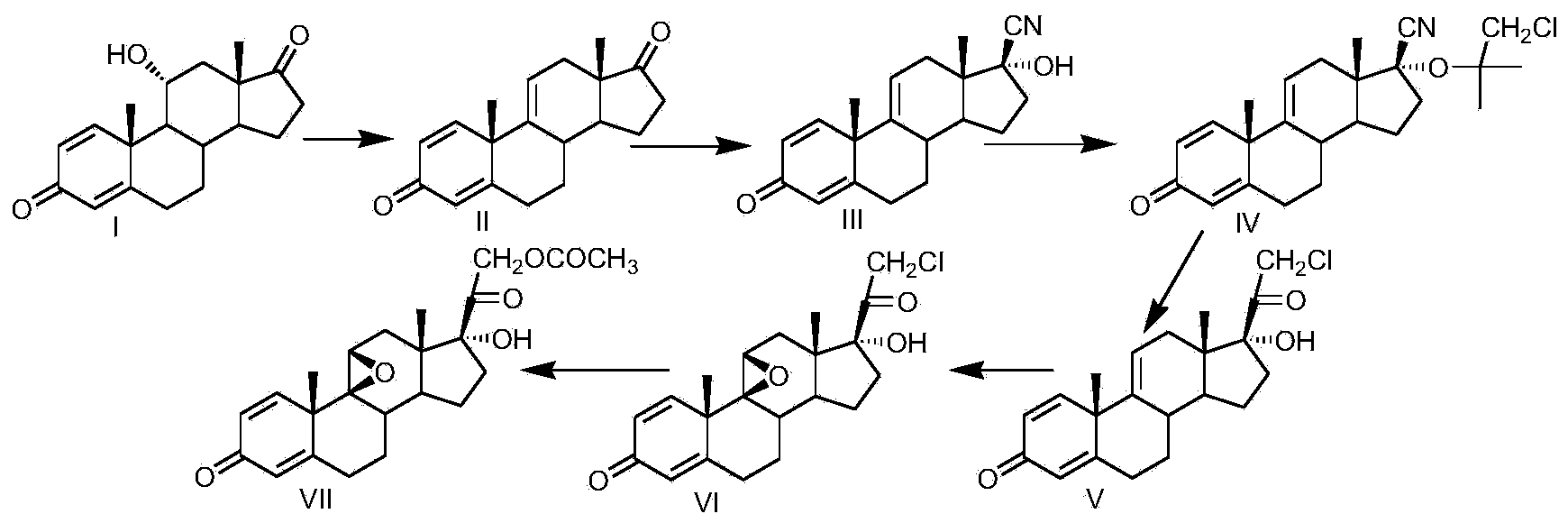
Preparation method for fluocinolone acetonide midbody
A technology of fluocinolone acetate and intermediates, applied in the directions of steroids, organic chemistry, etc., can solve the problems of eliminating many side reactions, prone to rearrangement, and low refining yield, etc., to improve yield and quality, and improve quality. and yield, the effect of reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Take a three-necked reaction flask and let it be protected by nitrogen. Add 300ml of 2-methylpyridine and 100g of compound I (11α-hydroxy-ADD) into the reaction flask. Stir at room temperature for 10-15 minutes, cool to -20°C, and add to the reaction solution in batches Add 20g of phosphorus pentachloride, stir for 10-15min, then pass 20g of sulfur dioxide into the reaction flask, keep the temperature at -20~-10℃, for about 1.5~2h, after the introduction, keep the temperature for 1h, TLC After the reaction was completed, 2500ml of water was slowly dropped into the reaction solution, filtered, washed with a small amount of water to neutrality, and the solid was dried at 60°C to obtain 92g of compound II, namely 1,4,9(11)-triene Androsta-3,17 dione, mass yield: 92%, HPLC purity: 98%.
[0031] Add 200ml of methanol, 100ml of acetone cyanohydrin, and 200.0g of compound II to a clean four-necked reaction flask. After stirring, add 200ml of 10wt% potassium carbonate aqueous solu...
Embodiment 2
[0037] Take a three-necked reaction flask and put it under nitrogen protection. Add 300ml of pyridine and 100g of compound I (11α-hydroxy-ADD) to the reaction flask. Stir at room temperature for 10-15 minutes, cool to 15°C, and add N-bromobutyl to the reaction solution. 54g of diimide, stirring for 10-15min, inject 20g of sulfur dioxide into the reaction flask, keep the temperature at 10-15℃, for about 1.5-2h, after the introduction, keep the reaction temperature for 1h, TLC detection, after the reaction is complete , Slowly drip 2500ml of water into the reaction solution, filter, wash with a small amount of water to neutrality, and dry the solid at 60°C to obtain 95g of compound II, namely 1,4,9(11)-trienandrosta-3, 17 diketone, mass yield: 95%, HPLC purity: 98%.
[0038] Add 300ml of acetone, 120ml of acetone cyanohydrin, and 200.0g of compound II to a clean and dry four-necked reaction flask. After stirring evenly, add 150ml of 10wt% sodium carbonate solution. The temperature ...
Embodiment 3
[0044] Take a three-necked reaction flask and put it under nitrogen protection. Add 400ml of diisopropylamine, compound I, 11α-hydroxy-ADD, to the reaction flask, stir at room temperature for 10-15 minutes, and cool to -10°C. Add N-chlorine to the reaction solution. Substitute succinimide 50g, stir for 10-15min, pass 20g sulfur dioxide into the reaction flask, keep the temperature below -10~-5℃, time about 1.5~2h, after passing through, keep the temperature and react for 1h, TLC It was detected that after the reaction was completed, 2500ml of water was slowly dropped into the reaction solution, filtered, washed with a small amount of water to neutrality, and the solid was dried at 60°C to obtain 94g of compound II, namely 1,4,9(11)-triene Androsta-3,17 dione, mass yield: 94%, HPLC purity: 98%.
[0045] Add 200ml methanol, 160g sodium cyanide, and 200.0g compound II to a clean four-necked reaction flask. The temperature of the system is controlled at 0~10℃. After stirring, 128ml o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com