Anti-paralichthys olivaceus immunoglobulin D monoclonal antibody as well as application and preparation method thereof
A monoclonal antibody and immunoglobulin technology, applied in the direction of immunoglobulin, antibodies, chemical instruments and methods, etc., can solve the problems of IgD deficiency, low content, limited to gene detection level, etc., and achieve the effect of novel design
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
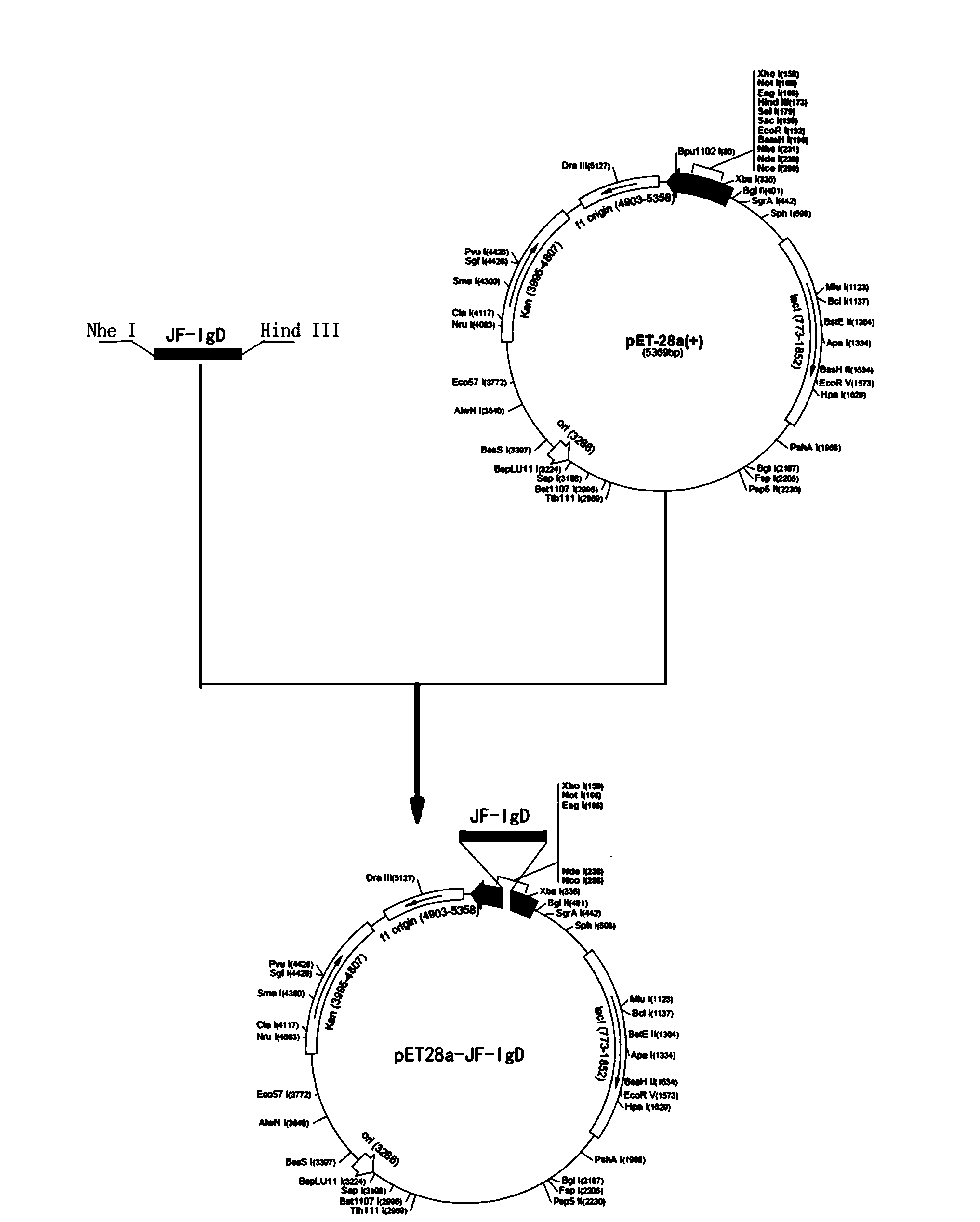
[0024] Example 1: Construction of a plasmid expressing the IgD heavy chain δ1-δ4 region of flounder and protein expression
[0025] (1) Peripheral blood cells of flounder were extracted with anticoagulant (6.7 mg heparin sodium and 0.5 g BSA per 50 ml of single cell suspension RPMI-1640) at a ratio of 1:1, and the percoll discontinuous density gradient centrifugation was used to obtain the flounder Peripheral blood leukocytes, the total RNA of leukocytes was extracted by Trizol method.
[0026] (2) According to the principle of primer design and the published full-length sequence of a flounder IgD heavy chain coding gene (GeneBank: AB052658), using the primer design software Primer5.0, combined with the characteristics of the multiple cloning site of the pET28(a) plasmid, choose Nhe I and Hind The restriction site of III was used as the insertion position of the target gene, and the expression primers for the δ1-δ4 region of the IgD heavy chain of flounder were designed:...
Embodiment 2
[0035] Example 2: Preparation of mouse anti-flounder IgD heavy chain δ1-δ4 region monoclonal antibody
[0036] 1. Immunity
[0037] Purified recombinant flounder IgD heavy chain protein was used as antigen. The dose of each immunization is 0.1ml, and the immunization is divided into 4 times. The interval between the first 2 immunizations is 2 weeks, and the interval between the next 3 immunizations is 1 week. The first two times are intraperitoneal injections, and the last two times are tail vein injections:
[0038] (1) Basic immunization: the purified recombinant protein is mixed with Freund's complete adjuvant in equal volume (V / V) as the antigen;
[0039] (2) Booster immunization: the purified recombinant protein was mixed with the same amount (V / V) of Freund's incomplete adjuvant as the antigen;
[0040] (3) Second booster immunization: purified recombinant flounder IgD heavy chain protein as antigen;
[0041] (4) Expansion immunization three days before fusion: puri...
Embodiment 3
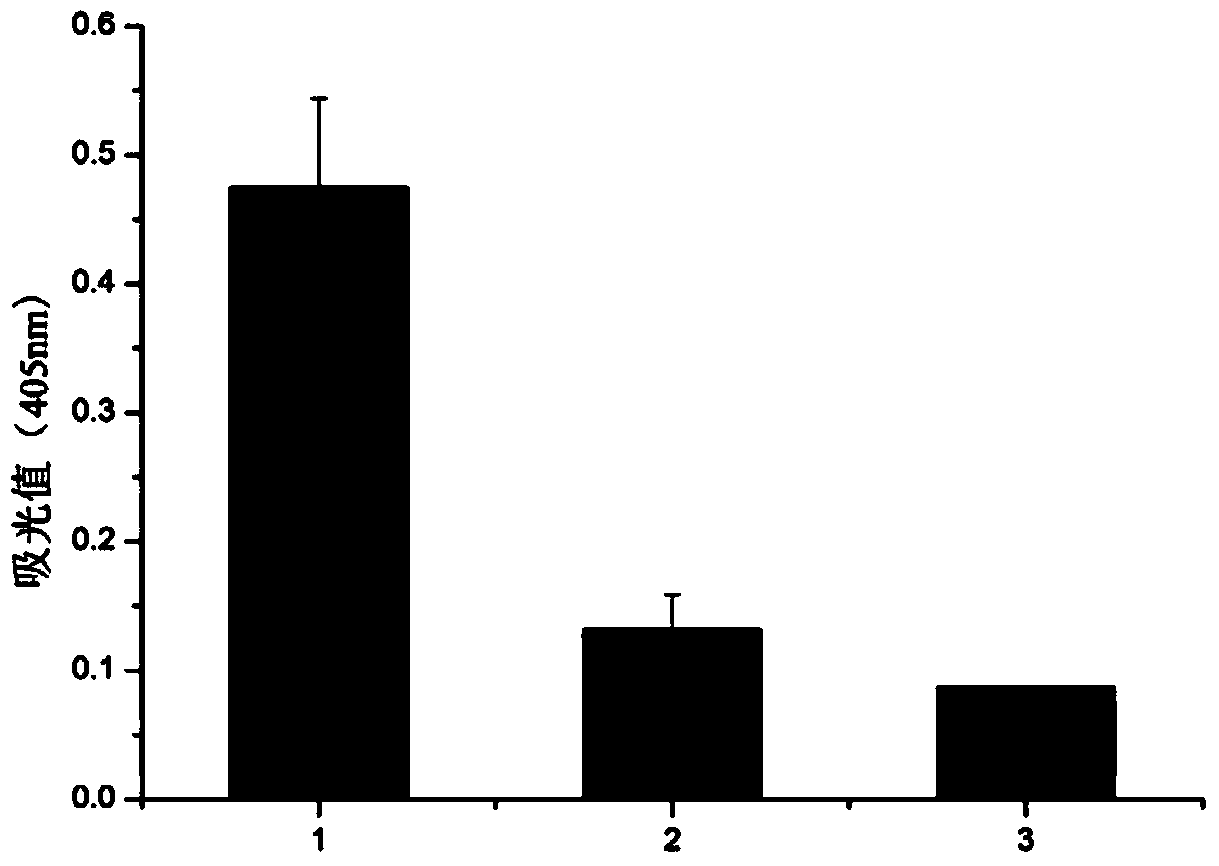
[0072] Example 3: Indirect enzyme-linked immunoassay identification of the monoclonal antibody of the present invention:
[0073] (1) Coating antigen: Dilute the purified recombinant flounder IgD heavy chain δ1-δ4 region protein with carbonate coating solution (pH 9.6) 1:10, add it to a 96-well microtiter plate (50 μl / well), Coating overnight at 4°C;
[0074] (2) Aspirate the coating solution, wash with PBST, 5min each time, three times;
[0075] (3) Add 200 μl 3% bovine serum albumin (with PBS) to each well and block at 37°C for 1 hour;
[0076] (4) Wash three times with the method (2);
[0077] (5) Add the culture supernatant of the hybridoma cells screened and cloned above as the primary antibody to the microtiter plate at 50 μl per well, and incubate in a 37°C incubator for 1 hour;
[0078] (6) Wash three times with method (2);
[0079] (7) Add alkaline phosphatase-labeled goat anti-mouse Ig (diluted 1:4000) as the secondary antibody to the microtiter plate at 50 μl pe...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com