Digoxin lipid microbubble and preparation method thereof
A technology of lipid microbubbles and octylolipids, which is applied in liposome delivery, pharmaceutical formulations, non-active ingredients of polymer compounds, etc., can solve the problems of rare research, low drug concentration, and low drug loading. Achieve the effects of enhancing immune function, reducing degradation, and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] 1), preparation of lecithin mixture
[0067] Egg yolk lecithin: glycerol: phosphate buffer solution is mixed uniformly at a ratio of 6:58:100, calculated by mass percentage.
[0068] 2), preparation of digoxigenin albumin nanoparticles
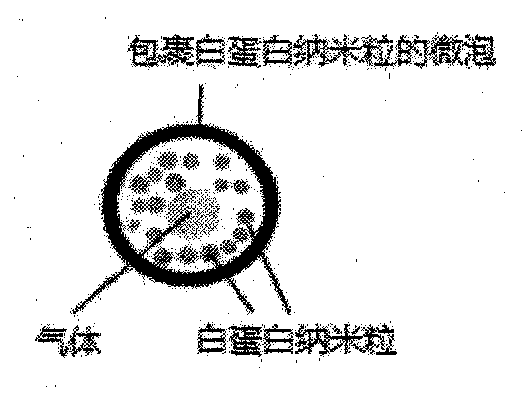
[0069] Digoxigenin albumin nanoparticles were prepared by desolvation method, accurately weighed 20mg of bovine serum albumin and dissolved in 2mL of water, and another 20mg of digoxin was dissolved in 12mL of absolute ethanol, and digoxigenin was dissolved at a volume flow rate of 1mL / min. Add octyl ethanol solution dropwise to albumin aqueous solution, add 100 mL of glutaraldehyde with a concentration of 0.25%, and stir in the dark for 4 hours to solidify, then remove ethanol by rotary evaporation at 35°C to obtain digoxigenin albumin nanoparticle suspension. See figure 1 . The encapsulation efficiency was 88.48% as measured by centrifugation.
[0070] 3), Preparation of Digoxigenin Nanoparticle Lipid Microbubbles
[0071] Take 4...
Embodiment 2-10
[0082] Examples 2-10 are operated according to the following parameters, and the others are the same as in Example 1, and the cumulative release rate experiment is detected under the condition of adding ultrasound.
[0083]
[0084]
[0085] Examples 8-10 of the present invention are comparative examples. Experimental results show that the type of phospholipids, the ratio of phospholipids, glycerol and phosphate buffer, and the selection of glutaraldehyde have a very important impact on the present invention.
[0086] The experimental results of Examples 1-7 of the present invention show that: through the cooperation of various parameters of the present invention, an encapsulation rate of 82.3% to 92% can be achieved, and the diameter of the prepared digoxin albumin lipid microbubbles is 4 to 7 Micron, in vitro and in vivo imaging effects are good, and the 24h in vivo drug release rate is 80-92%, which has a good application prospect.
Embodiment 11
[0088] The digoxin lipid microvesicles prepared in Example 1 are made into freeze-dried powder preparation
[0089] Pre-freezing: Put the subpackaged digoxin lipid microbubbles on the inner partition of the freeze-drying box. In the manual interface, set the temperature of the plate layer to 0°C for 1 minute (turn on the electric heating). After entering the box, Keep the product below 1°C for 60 minutes; turn on the two compressors, set the plate temperature at 40°C for 1 minute, and keep the product below -35°C for 60 minutes; set the plate temperature at -11°C for 60 minutes, and when the product reaches -11°C, Keep the product at -11°C for 60min; set the plate temperature at -38°C for 1min, and keep the product at -35°C for 60min.
[0090] Primary drying (sublimation drying)
[0091] Refrigerate the back box, and when the temperature of the back box reaches -40°C, turn on the vacuum pump, and then open the small butterfly valve after 2 seconds to evacuate the back box. W...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com