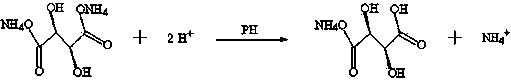Method for recycling and reusing L-(+)-tartaric acid in production of D-p-methyl sulfone phenyl ethyl serinate
A technology of tartaric acid and calcium tartrate, which is applied in the chemical field, can solve the problems that the product quality of L-(+)-tartaric acid cannot meet the split requirements, fail to meet the quality standard, and the product quality is poor, so as to achieve easy control and avoid side effects. Response, the effect of reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
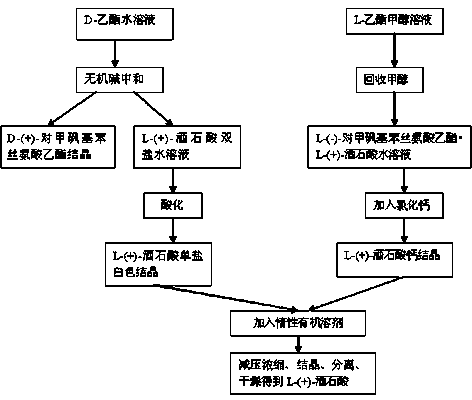
[0046] Production of L-(+)-tartaric acid by recovering L-(+)-diammonium tartrate from D-ethyl ester mother liquor
[0047] In a 5000L glass-lined reaction kettle, add pure water, start stirring, add the split D-ethyl ester·L-(+)-tartrate, add ammonia water dropwise to adjust the pH to 7~8, and obtain D-ethyl ester product and a mother liquor containing L-(+)-diammonium tartrate.
[0048] In a 5000L glass-lined reactor, add L-(+)-diammonium tartrate aqueous solution, stir, add hydrochloric acid dropwise to adjust pH=3.4 (control pH=3.3~3.6), cool, and control the temperature not to exceed 10~30°C. Freeze and cool to 10°C to crystallize, centrifuge, wash with cold water, and dry to obtain white crystals of high-purity L-(+)-ammonium hydrogen tartrate.
[0049] In a 5000L glass-lined reaction kettle, add 3000~4000kg of organic solvent acetone, stir, cool down to ≤10°C, add about 500~1000kg of L-(+)-ammonium hydrogen tartrate solid, cool down, and control the temperature in the...
Embodiment 2
[0054] Recovery of L-(+)-calcium tartrate from L-ethyl ester mother liquor to produce L-(+)-tartaric acid
[0055] L-(-)-p-thymphenylphenylserine ethyl ester · L-(+)-tartrate methanol solution is concentrated under reduced pressure to recover methanol, and water is added to obtain L-(-)-p-thymphenylphenylserine ethyl ester · L -(+)-tartaric acid aqueous solution is neutralized and freed by alkaline substances, and calcium chloride solid is added to react to form L-(+)-calcium tartrate crystals, cooled, separated and washed to obtain L-(+)-calcium tartrate crystals.
[0056] In the 5000L calcium tartrate recovery kettle, add D-ethyl ester free mother liquor or L-ethyl ester free mother liquor into the recovery kettle, inject 70~80% liquid level, start stirring; add high-purity calcium chloride solid in batches, and react Generate L-(+)-calcium tartrate crystals, cool to 10~25°C to crystallize, and centrifuge to obtain L-(+)-calcium tartrate.
[0057] In a 5000L glass-lined r...
Embodiment 3
[0062] Recovery of L-(+)-tartaric acid for application and separation
[0063] Add 2200~3000kg of anhydrous methanol into a 5000L split kettle, add 220Kg of L-(+)-tartaric acid recovered by the above process, heat up, and keep warm at 25~35°C for 30min~60min, until the L-(+)-tartaric acid is completely Dissolve and set aside; add the prepared 400Kg DL-(±)-p-thymphenylphenylserine ethyl ester methanol solution into the split kettle, stir for 40-60 minutes, heat up to 45-66°C and reflux, and keep warm for 30-60 minutes ;After the reaction is completed, cool down to 28-33°C and prepare for pressure filtration;
[0064] At the same time, preheat the diaphragm filter press to about 28-33°C and keep it warm. When the temperature of the split kettle is cooled to about 28-33°C, discharge the material to the filter press for filtration. After the discharge is completed, press, Drying with compressed nitrogen, unloading to obtain D-(+)-p-thymphenyl phenylserine ethyl ester L-(+)-tart...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com