Method for synthesizing ultraviolet-writable fluorine-containing erbium-containing polymer waveguide amplifier material
A waveguide amplifier and polymer technology, applied in the field of polymer near-infrared luminescent material synthesis, can solve the problems of complex device processing process and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Weigh 2g (0.005952mol) of hexafluorobisphenol A and dissolve it in about 20mL of THF, pour it into a three-neck flask placed in an ice bath, add 0.65g (0.006436mol) of triethylamine (Et 3 The amount of N should be slightly excess than the HCl generated in the reaction), and after stirring with nitrogen for 30 minutes, dissolve 0.5383g (0.006223mol) of acryloyl chloride in 10ml THF and quickly add it dropwise to the above solution, and leave the ice bath after reacting for 1 hour. The reaction was continued overnight at room temperature. The reaction solution was distilled under reduced pressure at 95°C in a rotary evaporator to remove tetrahydrofuran and triethylamine in the reaction system; 20g (0.2162mol) of epichlorohydrin was added, transferred to a three-necked flask with a constant temperature water bath at 50°C, and N 2 After 30min, add 0.25g of solid NaOH every 0.5h, add 12 times, a total of 3g; raise the temperature to 60°C, and react at a constant temperature ...
Embodiment 2
[0033] All the reaction raw materials and operating methods used are the same as in Example 1, except that the addition of erbium-containing complex EDPM is 0.3394g to obtain 0.3wt% fluorine-containing erbium-containing polymer with erbium content.
Embodiment 3
[0035] All the reaction raw materials and operating methods used are the same as in Example 1, except that the addition amount of the erbium-containing complex EDPM is 2.4934 g to obtain a fluorine-containing erbium-containing polymer with an erbium content of 1 wt%.
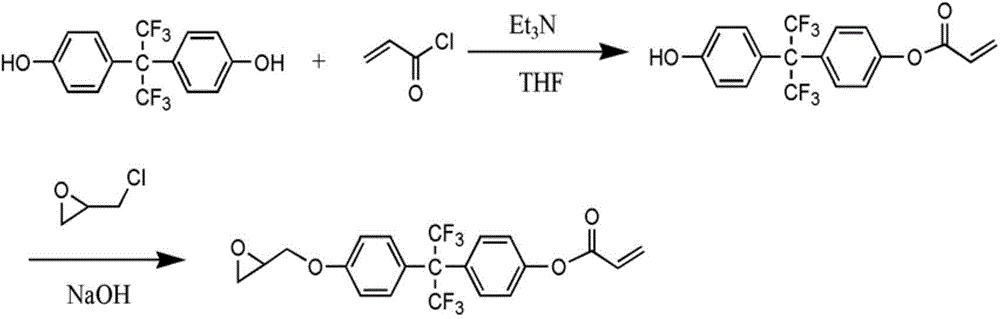
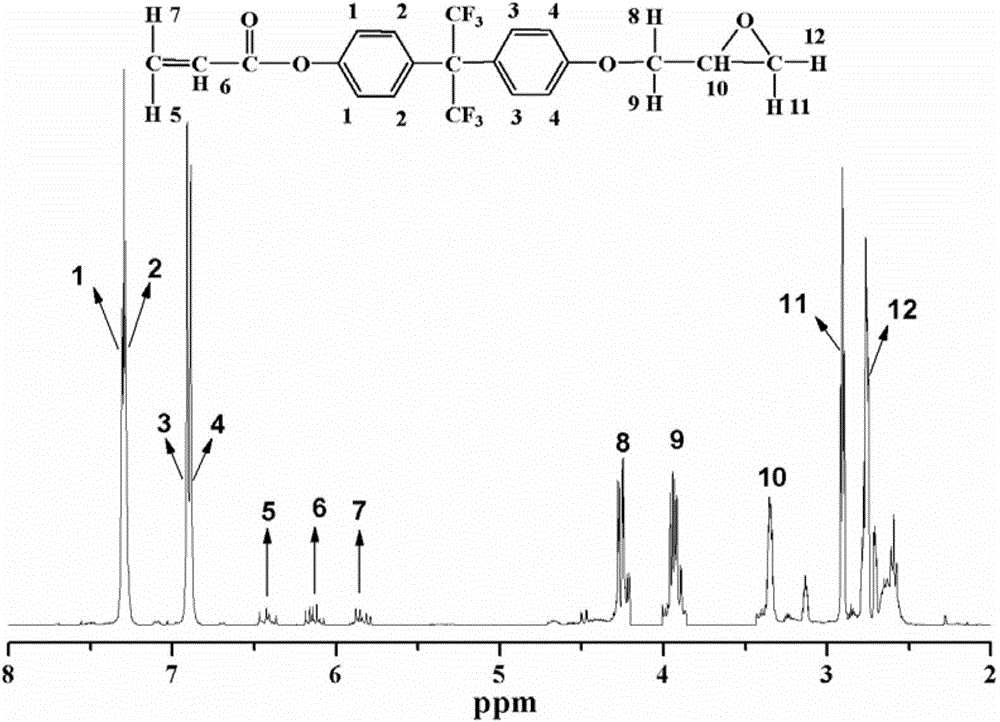
[0036] figure 1 Shown is the synthesis route of the fluorine-containing polymerizable monomer (FA) prepared in Example 1.
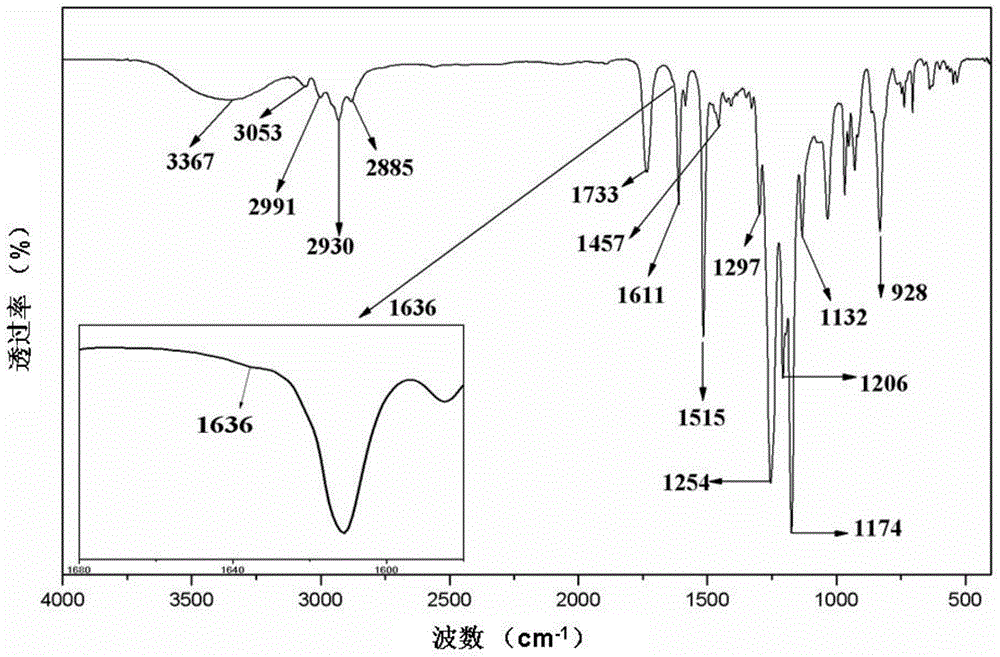
[0037] figure 2 Shown is the infrared spectrogram of the fluorine-containing active monomer (FA) prepared in Example 1. It can be seen from the figure that the reaction product of fluorine-containing bisphenol A and acryloyl chloride is at 1636cm -1 The characteristic peak of C=C double bond appeared at , and the 928cm -1 is the characteristic peak of the epoxy group, and 3367cm -1 The -OH absorption peak at the position basically disappears, which shows that the phenolic hydroxyl groups at both ends of the fluorinated bisphenol A have basically completed the reaction with acryloyl chl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com