Cobalt-base Fischer-Tropsch synthesis catalyst and preparation method and application thereof
A Fischer-Tropsch synthesis and catalyst technology, which is used in chemical instruments and methods, preparation of liquid hydrocarbon mixtures, catalysts for physical/chemical processes, etc. Activity and stability, enhanced wear resistance, uniform size distribution effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
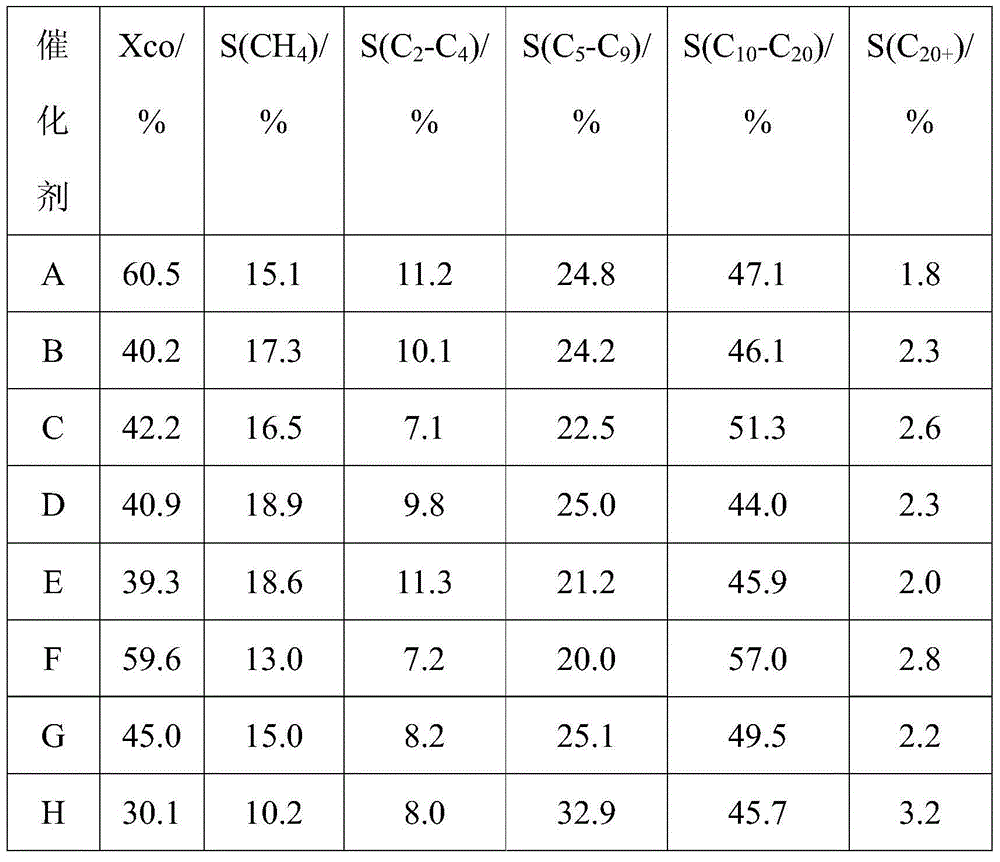
[0031] Catalyst A: includes 2wt% active component Co, and the rest is mesoporous carbon support; the active component Co is confined in the mesoporous carbon support.
[0032] The preparation method process of catalyst A is as follows:
[0033] 1) Weigh 6g of phenol and add it to a four-neck flask, dissolve it at 40°C, then add 3.2g of 20% sodium hydroxide and stir for 15min, then add 15.53g of 37wt% formaldehyde solution to a constant pressure funnel, then added dropwise to the flask, heated to 90°C, preferably to 70-95°C, more preferably to 80-90°C, after 50 minutes of reaction, transferred the solution to the beaker, cooled to room temperature, added Adjust the pH of the solution to neutral with nitric acid, and then dry it in a vacuum oven at 40°C for 24 hours to remove the water to obtain the phenolic resin, and then add an appropriate amount of absolute ethanol solution to it to prepare a phenolic resin solution with a mass fraction of 20%. ;
[0034] 2) Weigh 8gF127 a...
Embodiment 2
[0038] Catalyst B: includes 7wt% active component Co, and the rest is mesoporous carbon support and mesoporous material shell with high mechanical strength; the active component Co is confined in the mesoporous carbon support.
[0039] The preparation method process of catalyst B is as follows:
[0040] 1) Weigh 6g of phenol and add it to a four-necked flask, dissolve it at 43°C, then add 6.4g of sodium hydroxide with a mass fraction of 20%, stir for 10min, then add 25.87g of 37wt% formaldehyde solution to a constant Press the funnel, then add it dropwise to the flask, react at 80°C for 75 minutes, transfer the solution to a beaker, cool to room temperature, add nitric acid to adjust the pH of the solution to neutral, and then dry it in a vacuum oven at 50°C After 18 hours, remove the water to obtain the phenolic resin, then weigh an appropriate amount of absolute ethanol solution and add it to form a phenolic resin solution with a mass fraction of 18-22%, preferably 20%, wher...
Embodiment 3
[0046] Catalyst C: including 7wt% active component Co, and the rest is mesoporous carbon carrier and mesoporous material shell with high mechanical strength; the active component Co is confined in the mesoporous carbon carrier.
[0047] In the preparation method process of catalyst C, just the surfactant F127 in the step 2) of embodiment 2 is changed into the mixture of P123 and F127, and wherein P123 accounts for the massfraction of surfactant and is 25% (surfactant can adopt P123 , one or more of F127 or F108, preferably using P123 and F127), other steps are the same as in Example 2.
[0048] The specific surface area of the obtained catalyst C is 349m 2 / g, the average pore diameter is 5.0nm, the pore volume is 0.51mL / g, the thickness of the mesoporous material is 15um, and the average particle diameter is 150um.
[0049] Catalyst C is pre-reduced on a fixed bed before use, and the reducing gas is pure N 2 After the reduction is complete, the catalyst is transferred to ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific surface area | aaaaa | aaaaa |
| Average pore size | aaaaa | aaaaa |
| Pore volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com