Method for producing ion-conductive substance, ion-conductive substance, crystallized ion-conductive substance, and cell
A technology of ion conductivity and manufacturing method, which is applied in the field of manufacture of ion conductive materials, ion conductive materials, crystalline ion conductive materials and batteries, can solve the problems of reduced operating efficiency and low yield, and achieve high operating efficiency , High yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
manufacture example 1
[0177] [lithium sulfide (Li 2 S) Manufacture]
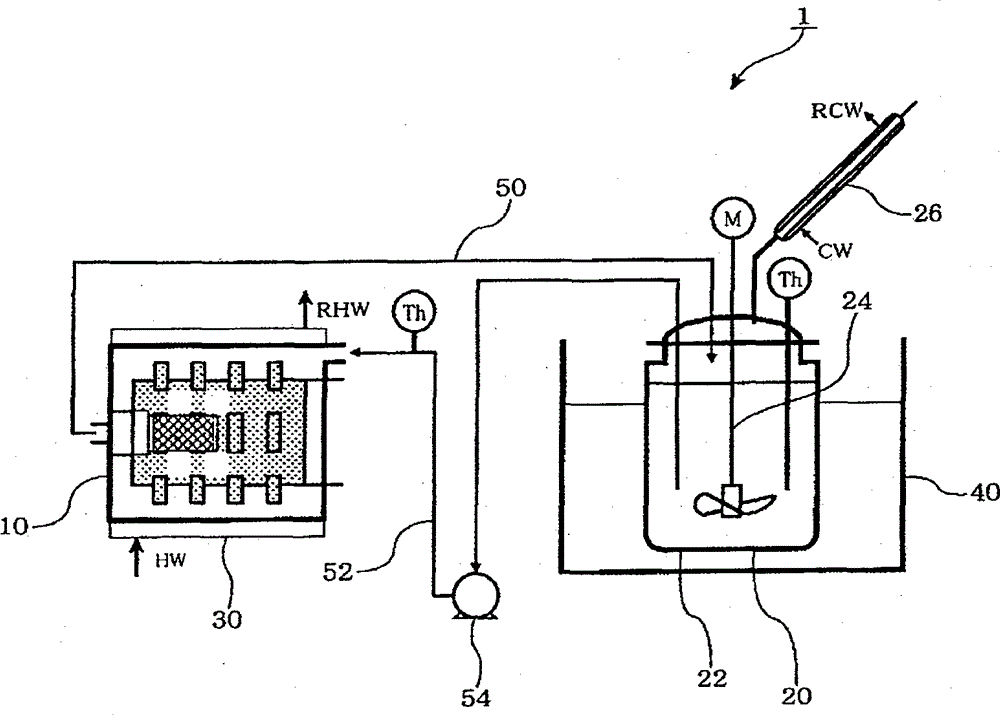
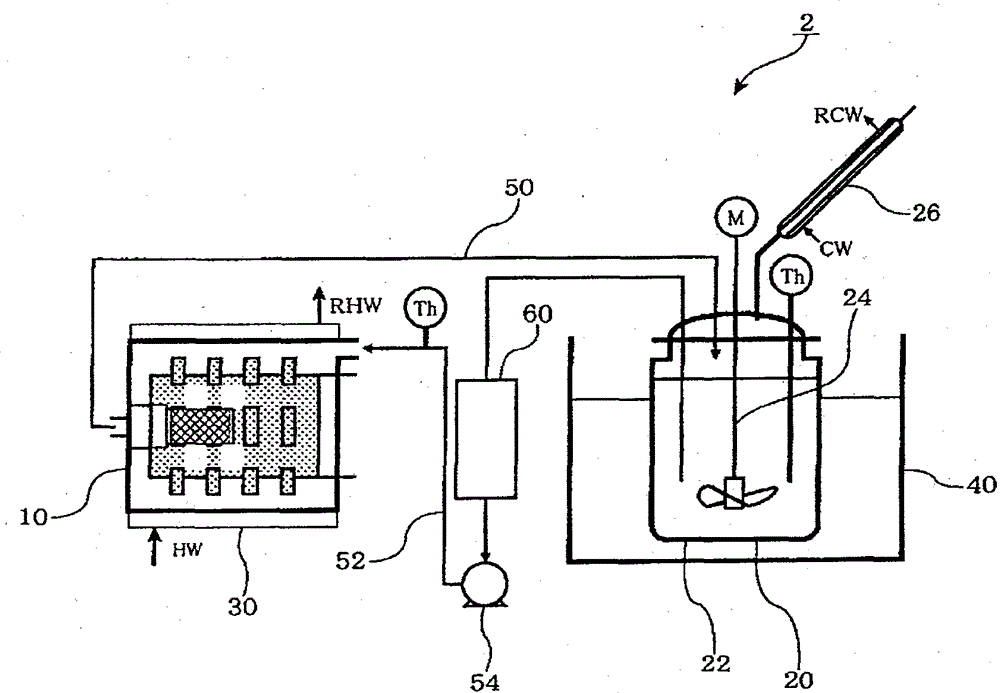
[0178] Under nitrogen flow, 270 g of toluene as a non-polar solvent was added to a 600 ml detachable flask, 30 g of lithium hydroxide (Honjo Chemical Co., Ltd.) was added, and it was kept at 95° C. while stirring at 300 rpm using a FULLZONE stirring blade. While blowing hydrogen sulfide into the slurry at a supply rate of 300 ml / min, the temperature was raised to 104°C. The azeotropic gas of water and toluene was continuously discharged from the separable flask. Dehydration is performed by condensing the azeotropic gas with a condenser outside the system. During this period, the same amount of toluene as distilled was continuously supplied to keep the level of the reaction liquid constant.
[0179] The water content in the condensate gradually decreased, and 6 hours after the introduction of hydrogen sulfide, no distillation of water was recognized (the water content was 22 ml in total). During the reaction, solids were dispe...
manufacture example 2
[0183] [micronization processing]
[0184] 26 g of lithium sulfide obtained in Production Example 1 was weighed into a Schlenk bottle in a glove box. 500 ml of dehydrated toluene (Wako Pure Chemical Industries, Ltd.) and 250 ml of dehydrated ethanol (Wako Pure Chemical Industries, Ltd.) were sequentially added thereto under a nitrogen atmosphere, and stirred with a stirrer at room temperature for 24 hours. After the reforming treatment, the bath temperature was raised to 120° C., and hydrogen sulfide gas was circulated at 200 ml / min for 90 minutes for treatment. After hydrogen sulfide gas treatment, the solvent was distilled off under nitrogen flow at room temperature, and then dried under vacuum at room temperature for 2 hours to recover micronized lithium sulfide.
[0185] Micronized lithium sulfide was evaluated in the same manner as in Production Example 1. The purity of lithium sulfide is 97.2%, the amount of lithium hydroxide is 0.3%, the average particle size is 9.1μm...
Embodiment 1
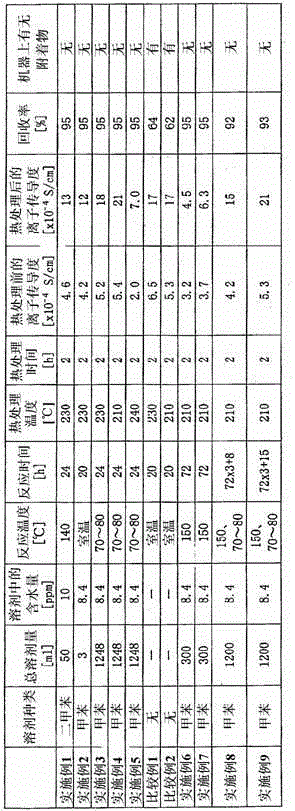
[0188] Lithium sulfide (LiS 2 ), so that the average particle size is 0.3 μm. The lithium sulfide 1.0g (64 mol%), phosphorus pentasulfide (P 2 S 5 ) (Aldrich Corporation) 1.61g (21 mol%) and lithium bromide (LiBr) (Aldrich Corporation) 0.42g (15mol%) were added to a flask with a stirrer replaced with nitrogen, and 50ml of di Toluene (Wako Pure Chemical Industries, Ltd.) was brought into contact at 140° C. for 24 hours.
[0189] The solid content was separated by filtration, and vacuum-dried at 120° C. for 40 minutes to obtain an ion conductive material (solid electrolyte). The recovery rate of the solid electrolyte was 95%. No deposits were found on the used flask or stirrer.
[0190] The ionic conductivity of the solid electrolyte is 4.6×10 -4 S / cm.
[0191] In addition, the particle diameter of lithium sulfide was measured using the laser diffraction and scattering type particle size distribution measuring instrument LMS-30 (Seishin Co., Ltd.).
[0192] In addition, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| ionic conductivity | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com