Preparation method of phorbol ester compound Euphorbia Factor L1
A technology of ester compound and Qianjinzi, which is applied in the field of preparation of diterpene alcohol ester compound Qianjinzi No. 1, can solve the problems of life-threatening, toxic and side effects, sequelae, iatrogenic diseases and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Preparation of Diterpene Alcohol Esters (QJZ-6)
[0032] 1. Obtaining the primary extract of QJZ-6
[0033] 1. Crushing:
[0034] Take Qianjinzi as a raw material, put it into a pulverizer for pulverization.
[0035] 2. Organic reagent extraction:
[0036] The stephenia powder was extracted with organic reagents, three consecutive extractions, each extraction time was 6h (heated to 60 degrees, and then heated for 6h). specific:
[0037] The first organic reagent extraction: add 2.5 kg of Stephania chinensis powder to 20 L of organic reagent, the organic reagent is ethyl acetate, stir well until the material liquid is uniform and there are no obvious lumps, turn on the water bath, extract for 6 hours, after the extraction is completed, collect the The supernatant was filtered with filter paper, and the supernatant was concentrated in a rotary evaporator and then transferred to a constant temperature blast drying oven at 60°C for drying to obtain the initial extract o...
Embodiment 2
[0043] Preparation of Diterpene Alcohol Esters (QJZ-6)
[0044] 1. Obtaining the primary extract of QJZ-6
[0045] 1. Crushing:
[0046] Take Qianjinzi as a raw material, put it into a pulverizer for pulverization.
[0047] 2. Organic reagent extraction:
[0048] The stephenia powder was extracted with organic reagents, three consecutive extractions, each extraction time was 6h (heated to 60 degrees, and then heated for 6h). specific:
[0049] The first organic reagent extraction: add 2.5 kg of Stephania chinensis powder to 20 L of organic reagent, the organic reagent is ethyl acetate, stir well until the material liquid is uniform and there are no obvious lumps, turn on the water bath, extract for 6 hours, after the extraction is completed, collect the The supernatant was filtered with filter paper, and the supernatant was concentrated in a rotary evaporator and then transferred to a constant temperature blast drying oven at 60°C for drying to obtain the initial extract of ...
Embodiment 3
[0056] Confirmation of QJZ-6 structure
[0057] 1. Purity analysis by HPLC:
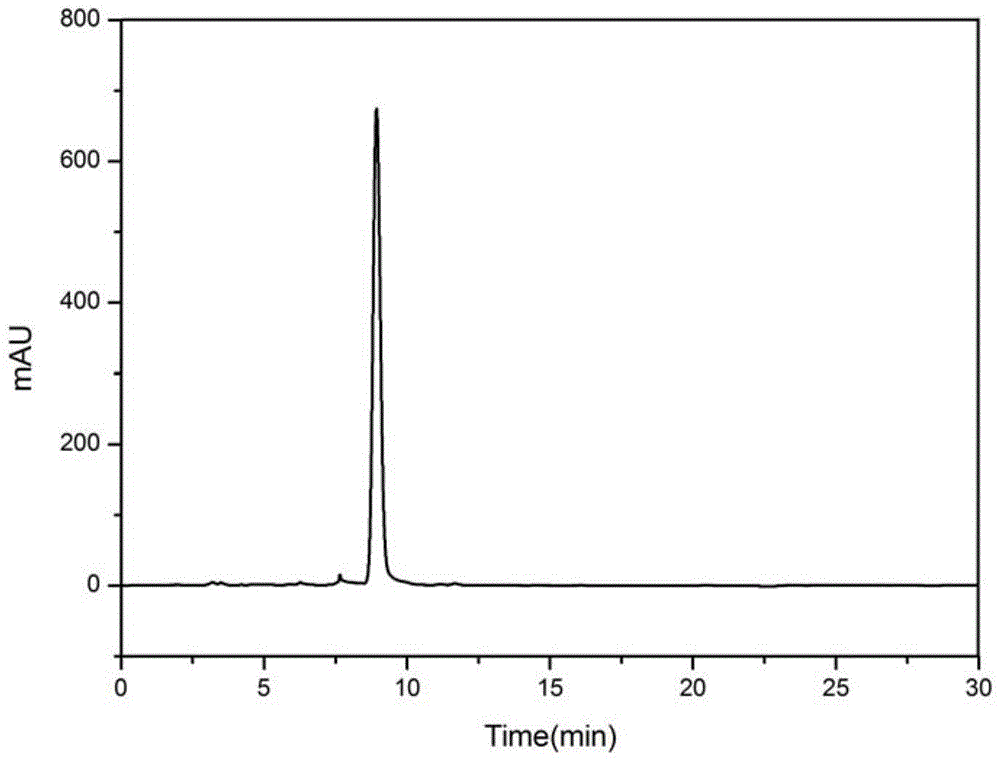
[0058] The QJZ-6 component (Example 2) was completely dissolved with 50% n-hexane-ethanol solution, configured into a 5 mg / mL solution, and analyzed for purity by HPLC. YMC normal phase chromatographic column (250mm×4.6mm, 5μm, ), the detection wavelength is 210nm, the detection conditions are: the mobile phase is n-hexane and ethanol, 98% n-hexane, 2% ethanol isocratic elution for 30min, the flow rate is 0.6mL / min, the injection volume: 5μL, the temperature is 30°C, the High performance liquid phase detection purity reaches 99%, see HPLC spectrogram figure 1 .
[0059] 2. NMR analysis:
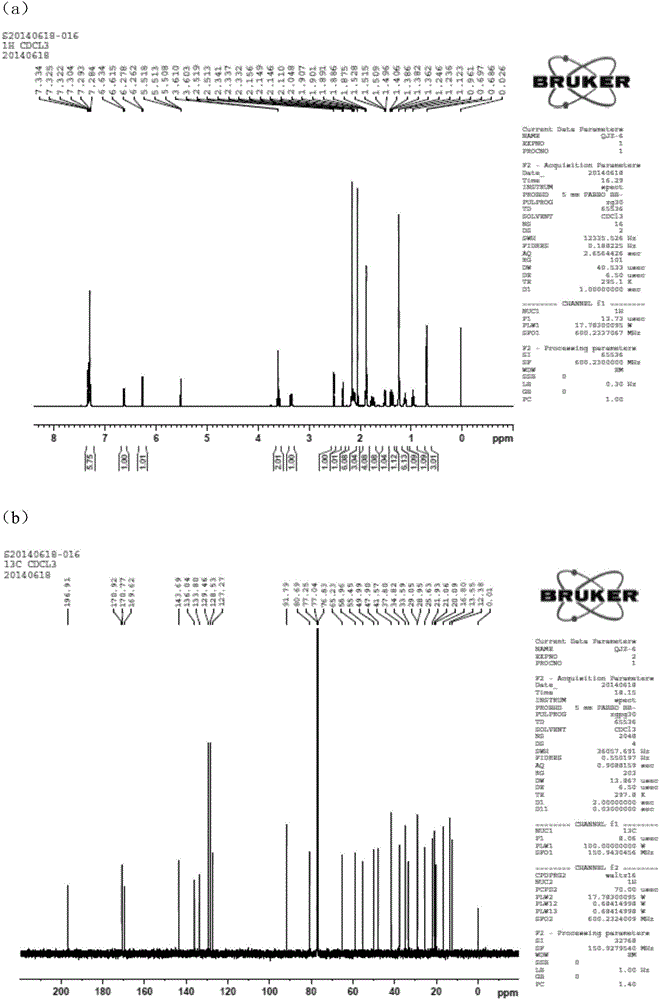
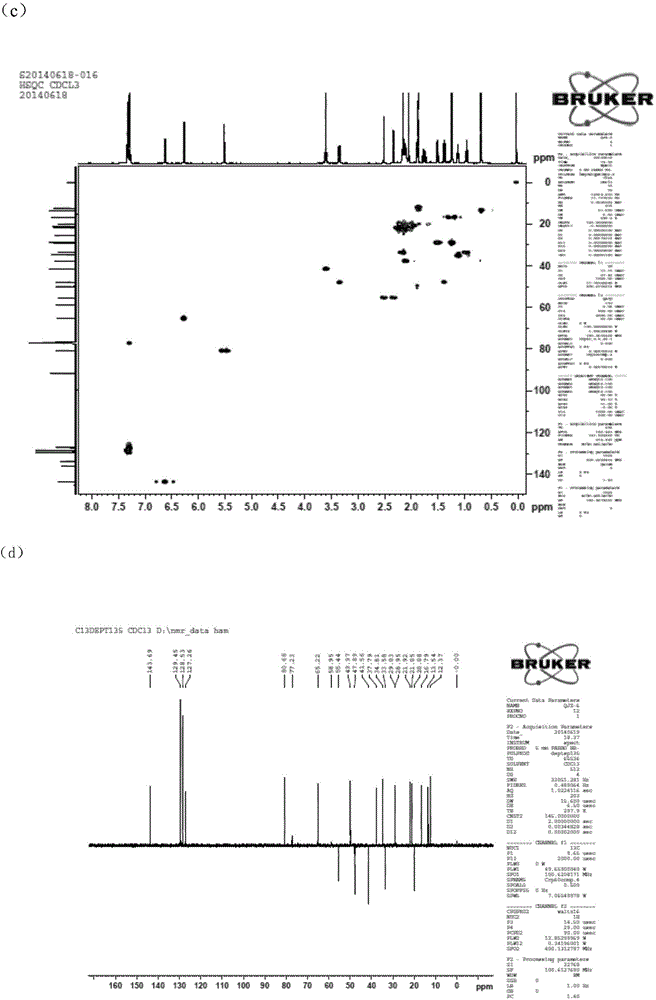
[0060] QJZ-6 was subjected to Bruker A.G AVI II 400PLUS, Hanmeng Biotechnology (Tianjin) Co., Ltd., and the NMR spectra were as follows figure 2 shown. The NMR data of QJZ-6 obtained from 1H NMR, 13C NMR, and HMBC spectra are shown in Table 1.
[0061] Table 1: 1H NMR (400MHz, J in Hz) and 13C NMR (100MHz) ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com