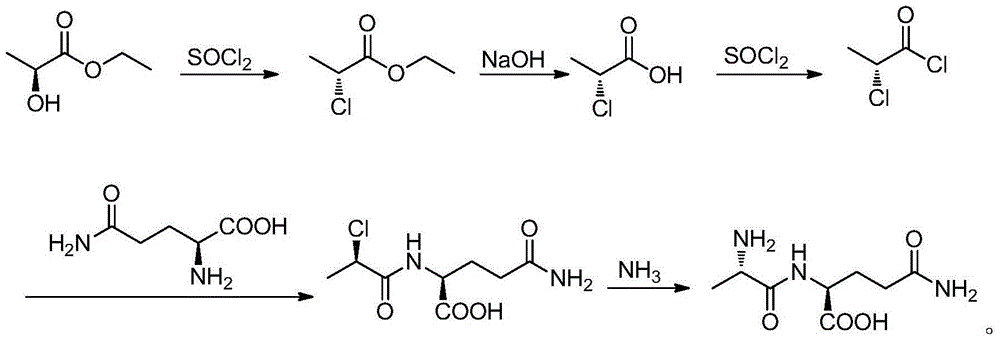
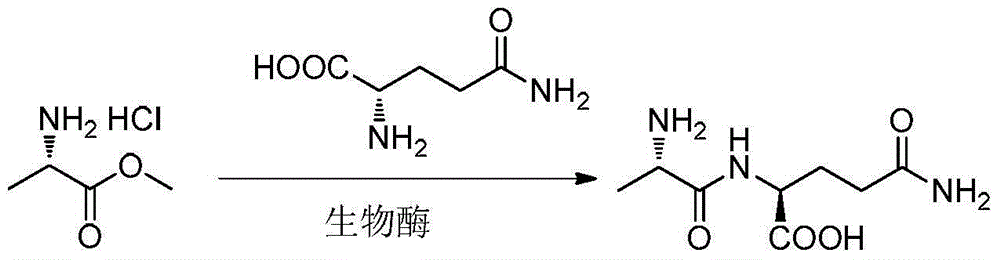
Bio-enzyme for synthesis of N(2)-L-alanyl-L-glutamine by catalysis as well as preparation method and application thereof
A technology of glucuronide dipeptide and bio-enzyme, which is applied in the field of catalyzing and synthesizing glucoglutide dipeptide and its preparation, can solve the problems of high process cost, long reaction synthesis route, and large environmental pollution, and achieve simple equipment requirements, high solvent recycling rate, Post-processing simple effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] Embodiment 1: the genetic engineering strain construction of biological enzyme
[0071] The biological enzyme gene fragment synthesized by the whole gene (sequence shown in SEQ ID NO: 1, synthesized by Nanjing GenScript Biotechnology Co., Ltd.), was subjected to restriction endonucleases EcoRI and Not I (purchased from New England Biolabs Company, Operate according to the instructions) after enzyme digestion, recombine into yeast expression vector pPIC9K (invitrogen company), transform into E.coli TOP10 (purchased from Beijing Quanshijin Biotechnology Co., Ltd.), put E.coli TOP10 in LB liquid medium Incubate overnight at 37°C with shaking at 160 rpm, and extract the recombinant plasmid. Using the restriction endonuclease SalI (purchased from New England Biolabs company, operate according to the instructions) to linearize the recombinant plasmid.
[0072] The preparation of Pichia pastoris GS115 competent cell (invitrogen company): Pichia Pastoris GS115 single bacteriu...
Embodiment 2
[0075] Embodiment 2: the fermentation preparation of biological enzyme
[0076] Streak the original strain on the YPD plate, and culture it upside down overnight at 30°C. Pick a single colony (1 mm in diameter) from the plate and put it in 50 ml of YPD liquid medium (10 g of yeast powder, 10 g of peptone, 10 g of glucose, add water to make up to 1 L), shake overnight at 30°C and 200 rpm for 24 hours. OD600 grows to 4-5. Inoculate 10% of the inoculum into 300ml YPD liquid medium (1L Erlenmeyer flask) shaker flask, shake culture at 30°C and 200rpm on a shaker, and the OD600 grows to about 12 after about 24h. Will Fermentation medium After the material is configured per liter, it is poured into a fermenter (30L), and sterilized at 121°C for 30 minutes; after cooling down, the temperature is controlled at 30°C, and the pH value is adjusted to 5.0 with ammonia water. Inoculate the well-grown seed solution into the tank, and the inoculum amount is 5%. Adjust the rotation speed ...
Embodiment 3
[0078] Add 58g of L-glutamine (397mmol) into 400mL of water, cool down to 10°C, adjust the pH to 9.0 with NaOH aqueous solution (2M), add 110mg of dry enzyme powder into the reaction system, and then add L-alanine methyl ester aqueous solution (Dissolve 82g of L-alanine methyl ester hydrochloride (590mmol) in 100mL of water) dropwise into the reaction system, while adding dropwise NaOH aqueous solution (2M) to keep the pH at 8.9-9.0; when the pH remains unchanged, the reaction At the end, heat up to 60°C to inactivate for 30 minutes, add 20g of activated carbon, stir for 10 minutes and then filter; the filtrate is desalted by nanofiltration, and distilled under reduced pressure until a large amount of solids are precipitated; the temperature is lowered and filtered, and the solids are dried under reduced pressure at 50°C to obtain crude dipeptide 60g.
[0079] Refining: Dissolve 60g of crude dipeptide in 60mL of water, add 300mL of methanol at 50°C, slowly cool down to 5-10°C ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com