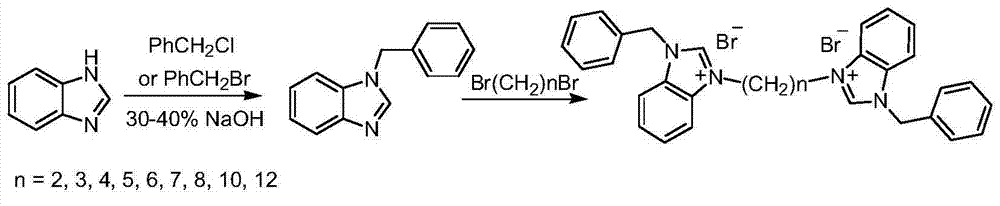
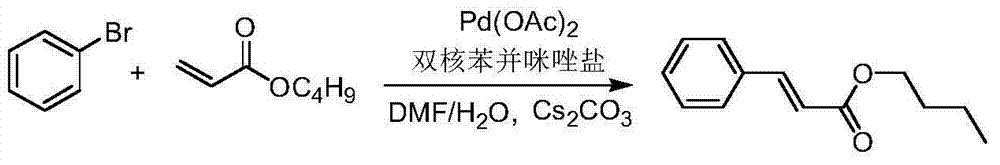
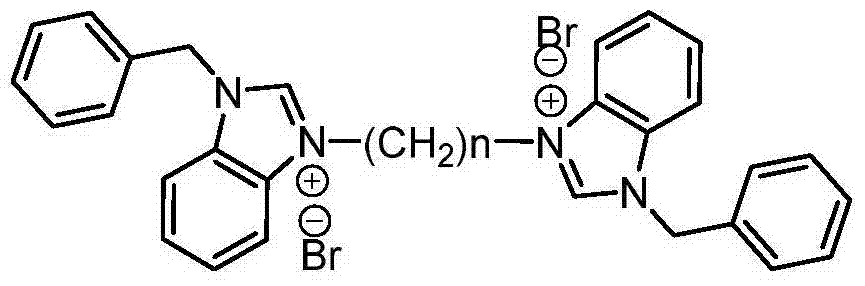Dicaryon benzimidazole ionic salt as well as preparation method and application thereof
A technology of nuclear benzimidazole and benzimidazole, which is applied in the field of binuclear benzimidazole ion salt and its preparation, and achieves the effects of high yield, strong coordination ability and good product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Synthesis of N-benzylbenzimidazole:
[0021] In a 250mL three-necked round-bottomed flask with a reflux condenser, add 8.85g (0.075mol) benzimidazole, 40mL 40% NaOH solution and 3.22g (0.01mol) tetrabutylammonium bromide successively, after magnetic stirring for 30min, 11.27 mL (0.098 mol) of benzyl chloride was slowly added dropwise. After the dropwise addition, the reaction temperature was raised to the slight reflux of the system, and the reaction was continued for 8 hours. Cool the reaction mixture to room temperature, filter with suction, dissolve the filter cake in 60 mL of ethyl acetate and 45 mL of water, and separate the organic phase with a separatory funnel. After the organic phase was dried over anhydrous sodium sulfate, the solvent was spun off under reduced pressure to obtain the crude product as a tan solid. The crude product was recrystallized by adding 6 mL of toluene to obtain 10.3 g of a light yellow solid with a yield of 66% and a melting point of ...
Embodiment 2
[0023] Synthesis of N-benzylbenzimidazole:
[0024] Add 8.85g (0.075mol) benzimidazole, 40mL30%NaOH solution and 3.00g tetrabutylammonium chloride successively in the three-necked round-bottomed flask that has reflux condenser in 250ml, after magnetic stirring 30min, slowly add dropwise 11.65mL ( 0.098 mol) benzyl bromide. After the dropwise addition was completed, the reaction temperature was raised to the slight reflux of the system, and the reaction was continued for 8 hours. Cool the reaction mixture to room temperature, filter with suction, dissolve the filter cake in 60 mL of ethyl acetate and 45 mL of water, and separate the organic phase with a separatory funnel. The organic phase was dried by adding anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure to obtain a tan solid. The crude product was recrystallized by adding 6 mL of toluene to obtain 10.78 g of a light yellow solid with a yield of 69% and a melting point of 116-117°C. 1 H N...
Embodiment 3
[0026] Synthesis of 3,3'-dibenzyl-1,1'-ethylenedibenzimidazolium dibromide:
[0027] Add 5.00g (0.024mol) 1-benzylbenzimidazole, 1.03mL (0.012mol) 1,2-dibromoethane and 60mL toluene to a 250mL three-necked round-bottomed flask equipped with a reflux condenser. The system was heated up to 100°C, and reacted for 72 hours under magnetic stirring. After the reaction was completed, the system was fully cooled. After rapid filtration, the filter cake was recrystallized from a mixed solution of 1 mL of acetonitrile, 25 mL of ethyl acetate and 5 mL of diethyl ether, and then recrystallized with absolute ethanol. 5.51 g of white solid was obtained with a yield of 76%. 1 H NMR (400MHz, TMS, CDCl 3 )δ: 10.11(s, 2H), 8.19(d, J=6.0Hz, 2H), 8.00(d, J=6.0Hz, 2H), 7.59(t, J=6.0Hz, 4H), 7.52-7.50( m,10H),5.84(s,4H),5.03(t,J=6.0Hz,4H); 13 C NMR (100MHz, DMSO) δ: 146.57, 134.60, 134.05, 132.36, 132.17, 131.73, 131.54, 130.29, 130.15, 117.37, 116.45, 53.25, 51.49; MS m / z: 443.3(M + ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com