pH-sensitive polymer micelles for drug delivery
A polymer glue and drug technology, which can be used in drug combinations, inactive components of polymer compounds, anti-tumor drugs, etc., can solve problems such as limiting drug absorption, increasing drug transmembrane absorption, and tumor cell drug resistance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0172] Example 1: PLA 2K -NH 2 Synthesis
[0173] Put 10g of lactide in a 250mL reaction flask, add 150mL of anhydrous toluene, heat and stir to 100°C to dissolve the lactide. Weigh 0.71 g of 3-(tert-butyloxycarbonylamino)-1-propanol, and dissolve it in an appropriate amount of anhydrous toluene. Weigh 0.1g stannous octoate (Sn(Oct) 2 ), and dissolved in an appropriate amount of anhydrous toluene. Under the condition of nitrogen protection, the above two mixed solutions were added into the lactide-toluene solution, under nitrogen protection, stirred at 100°C, and reacted for 10 hours. After 10 hours, the toluene was removed under reduced pressure, the product was dissolved in anhydrous dichloromethane, precipitated in anhydrous methanol, and the flocculent precipitate obtained by centrifugation was vacuum-dried, and the obtained white colloidal solid was PLA-NH-Boc. Yield about 65%.
[0174] Dissolve 1 g of PLA-NH-Boc obtained above in 12 mL of anhydrous dichlorometha...
Embodiment 2
[0175] Embodiment 2: the synthesis of BLG-NCA
[0176] Put 10 g of glutamic acid-γ-benzyl ester (BLG) in a 100 mL reaction flask, add 30 mL of anhydrous tetrahydrofuran, and stir at 50°C. Weigh 6g of triphosgene (BTC), add it into the reaction bottle, and stir at 50°C for about 3 hours. The reaction solution was poured into 150 mL of anhydrous n-hexane, refrigerated at -20°C for 48 hours, and filtered to obtain the crude BLG-NCA. The crude BLG-NCA was recrystallized three times from anhydrous ethyl acetate and anhydrous n-hexane, and dried under reduced pressure to obtain white needle crystals as the final product BLG-NCA. The yield is about 87%.
Embodiment 3
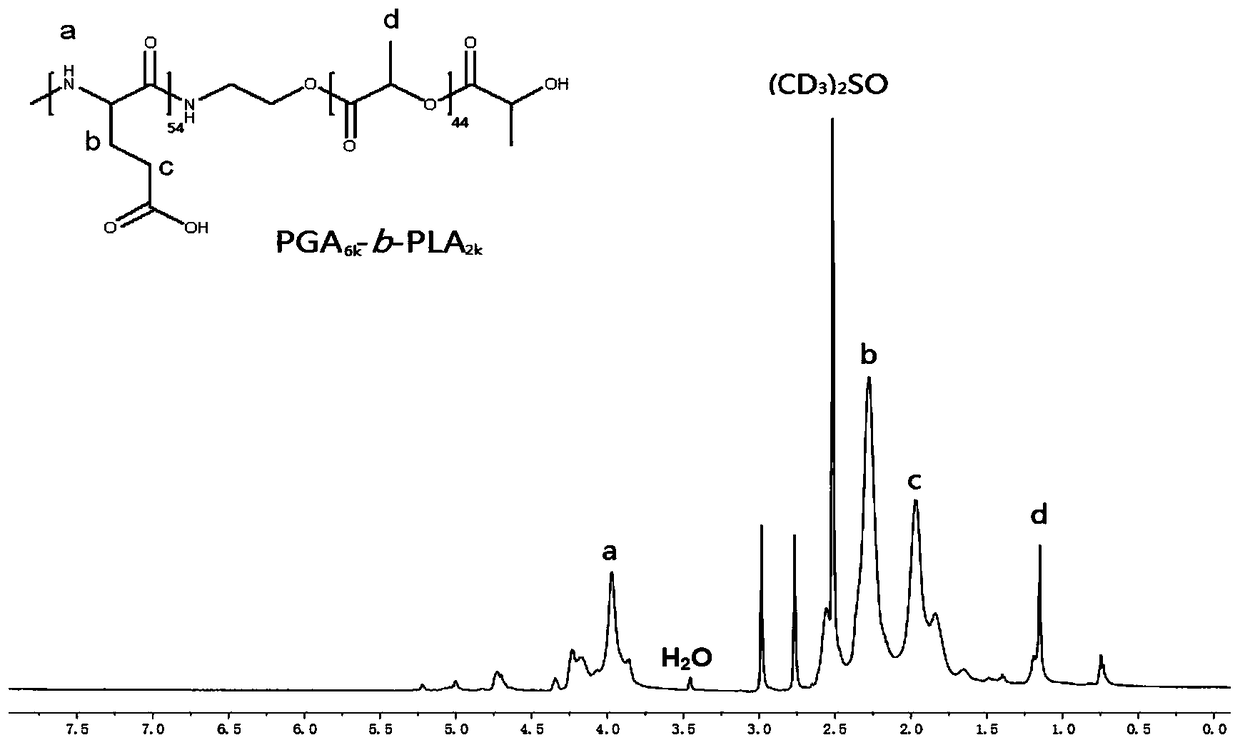
[0177] Example 3: PGA 6K -b-PLA 2K Synthesis
[0178] Step 1: Place 3 g of the BLG-NCA obtained in Example 2 into a 250 mL reaction flask and dry under reduced pressure for 2 hours, add 150 mL of anhydrous DMF under nitrogen protection, and then add triethylamine (the amount is 0.075 mole times the amount of BLG-NCA ). Take by weighing 0.15g embodiment 1 gained PLA 2K -NH 2 , added to the reaction flask under the protection of nitrogen, and stirred at room temperature for 72 hours. The reaction solution was poured into excess anhydrous ether for precipitation, filtered, and dried under reduced pressure to obtain PBGA-b-PLA as a pale yellow solid. The yield of step 1 was 92%.
[0179] Step 2: Dissolve 1 g of PBGA-b-PLA obtained in the above steps in 10 mL of dichloroacetic acid, add 5 mL of HBr (33%), stir at room temperature for 2 hours, then drop the reaction solution into excess -20°C ether to settle, wash, Filtration, the obtained white solid was dissolved in DMF,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com