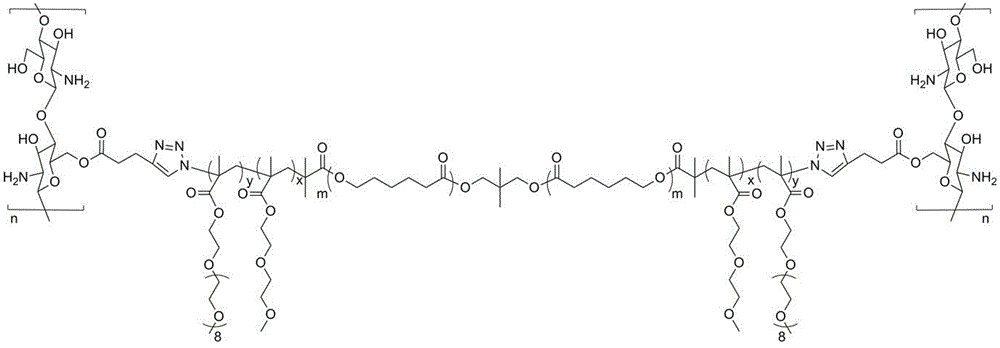
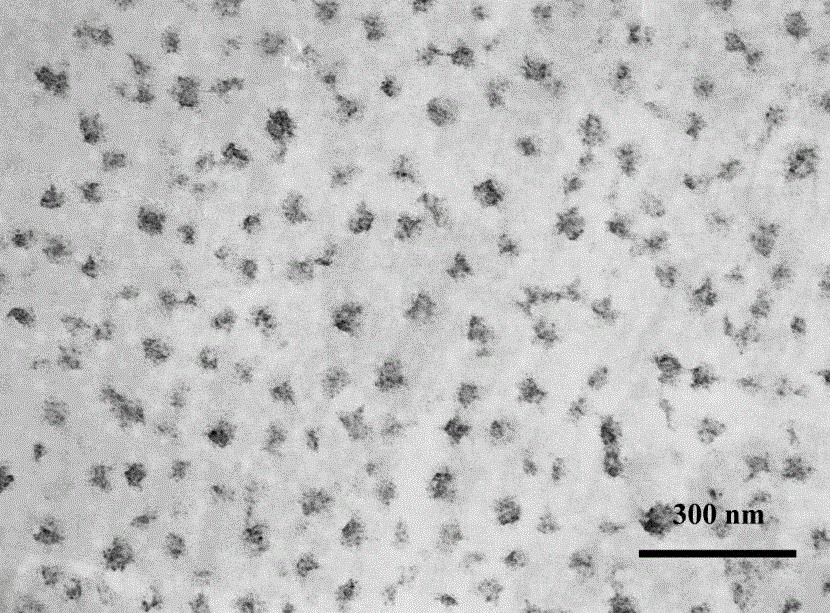
Preparation method of temperature response type polymer for controlled drug release and genetic vectors
A temperature-responsive, gene carrier technology, applied in the fields of polymer materials and biomedical engineering, can solve the problems of low transfection rate, limited application, high molecular weight, and achieve improved transfection efficiency, good application prospects, and simple and feasible synthesis methods. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] 20.0g chitosan oligosaccharides and 51.9g phthalic anhydride were dissolved in 150ml N,N-dimethylformamide, the system was refrigerated and evacuated three times, reacted at 120°C under argon protection for 8h, and then cooled by methanol precipitation Washed, filtered and dried in vacuum to obtain product I phthalylated chito-oligosaccharides; 15.0g product I, 27.7g 4-pentynoic acid, 54.1g 1-ethyl-(3-dimethylaminopropyl) )Carbonimide hydrochloride and 19.1g hydroxybenzotriazole are dissolved in 150ml N,N-dimethylformamide, the system is frozen and vacuumed three times, reacted at room temperature under argon protection for 24h, and precipitated by methanol Repeatedly dissolve the precipitate, filter and dry under vacuum to obtain product II alkynylated-phthalylated chito-oligosaccharide; dissolve 10.0g product II and 6.0g hydrazine hydrate in 50ml N,N-dimethylformamide, The system was refrigerated and evacuated three times, and reacted at 100°C for 4 hours under argon p...
Embodiment 2
[0037] Dissolve 10.0g chitosan oligosaccharide and 34.6g phthalic anhydride in 80ml N,N-dimethylformamide, freeze the system and evacuate three times, react at 100°C for 12h under the protection of nitrogen, and then precipitate and wash with methanol after cooling , After filtering, vacuum drying to obtain product I phthalylated chitosan oligosaccharide; 7.5g product I, 11.5g 4-pentynoic acid, 22.5g 1-ethyl-(3-dimethylaminopropyl) Carbonimide hydrochloride and 8.0g hydroxybenzotriazole were dissolved in 80ml N,N-dimethylformamide, the system was frozen and vacuumed three times, reacted at room temperature under nitrogen protection for 24h, precipitated by methanol and dissolved repeatedly Precipitate, filter and dry in vacuum to obtain product II alkynylated-phthalylated chito-oligosaccharide; dissolve 5.0 g product II and 4.8 g hydrazine hydrate in 50 ml N,N-dimethylformamide, and freeze the system Vacuum three times, react at 120°C for 6 hours under the protection of nitroge...
Embodiment 3
[0041] Dissolve 15.0g chitosan oligosaccharide and 86.5g phthalic anhydride in 100ml N,N-dimethylformamide, freeze the system and evacuate three times, react at 150℃ for 8h under nitrogen protection, then precipitate and wash with methanol after cooling , After filtering, vacuum drying to obtain product I phthalylated chitosan oligosaccharide; 10.0g product I, 15.3g 4-pentynoic acid, 30.0g 1-ethyl-(3-dimethylaminopropyl) Carbonimide hydrochloride and 10.7g hydroxybenzotriazole were dissolved in 120ml N,N-dimethylformamide, the system was frozen and vacuumed three times, reacted at room temperature under nitrogen protection for 36h, precipitated by methanol and dissolved repeatedly Precipitate, filter and dry in vacuum to obtain product II alkynylated-phthalylated chito-oligosaccharides; dissolve 6.0g product II and 5.7g hydrazine hydrate in 80ml N,N-dimethylformamide, and freeze the system Vacuum three times, react at 100°C for 8 hours under the protection of nitrogen, after co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com