Method for preparing dichloroaniline by continuously catalyzing and hydrogenating dichloronitrobenzene
A technology for dichloronitrobenzene and catalytic hydrogenation, which is applied in the preparation of amino compounds, chemical instruments and methods, preparation of organic compounds, etc. The effect of preventing dechlorination side reactions, good use effect and high reaction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
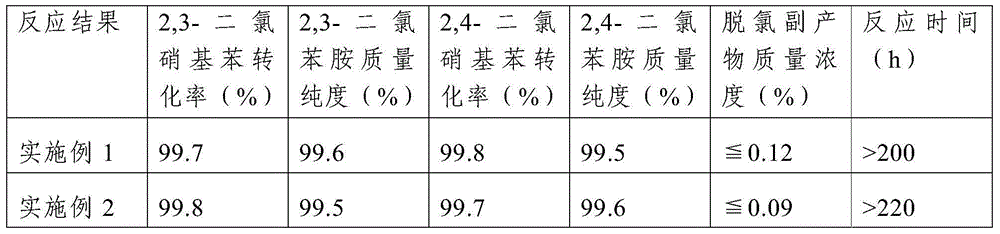
Embodiment 1
[0025] This embodiment includes the following steps:
[0026] Step 1. Load the catalyst in a fixed-bed reactor, and pass a reducing gas into the fixed-bed reactor filled with the catalyst under normal pressure, and then increase the temperature of the fixed-bed reactor to 260°C. Reduction treatment; the catalyst includes a carrier, platinum and metal promoters supported on the support, the mass content of platinum in the catalyst is 0.6%, the mass content of the metal promoter is 0.2%, the support is activated carbon, and the metal The auxiliary agent is tin; the specific surface area of the activated carbon is 1500m 2 / g, the particle size is 1mm; the reducing gas is hydrogen diluted with nitrogen, the volume content of hydrogen in the reducing gas is 15%, the flow rate of the reducing gas is 100 mL / min, and the filling volume of the catalyst Is 10mL, and the time of the reduction treatment is 8h;
[0027] Step 2. Increase the volume content of hydrogen in the reducing gas desc...
Embodiment 2
[0030] Example 2 is the same as Example 1, except that: in step 1, the mass content of platinum in the catalyst is 0.3%, the mass content of the metal promoter is 0.05%, and the metal promoter is vanadium.
[0031] Table 1 Catalytic hydrogenation reaction results of Example 1 and Example 2
[0032]
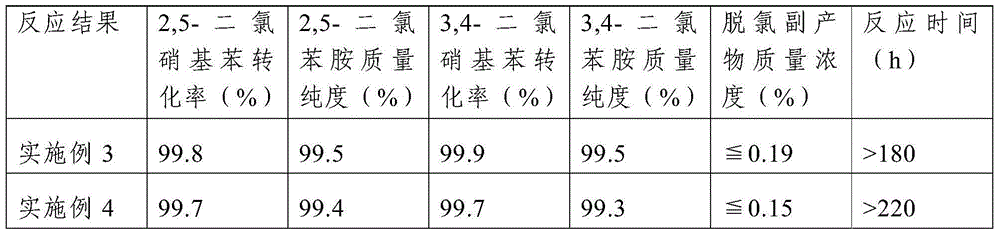
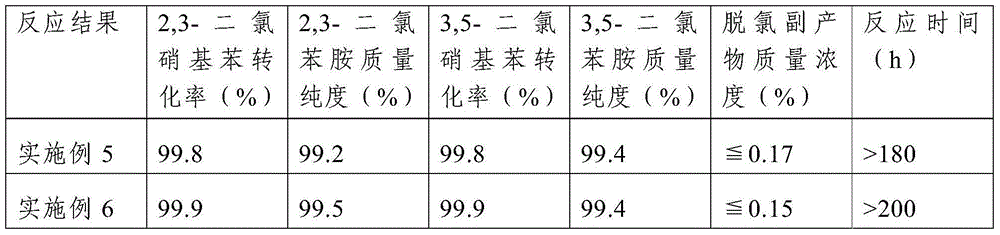
Embodiment 3
[0034] This embodiment includes the following steps:
[0035] Step 1. Load the catalyst in a fixed-bed reactor, pass reducing gas into the fixed-bed reactor filled with the catalyst under normal pressure, and then increase the temperature of the fixed-bed reactor to 200°C for the catalyst Reduction treatment; the catalyst includes a carrier, platinum supported on the carrier and a metal promoter, the mass content of platinum in the catalyst is 0.8%, the mass content of the metal promoter is 0.3%, the carrier is activated carbon, and the metal The auxiliary agent is tin; the specific surface area of the activated carbon is 1200m 2 / g, the particle size is 1.5mm; the reducing gas is hydrogen diluted with nitrogen, the volume content of hydrogen in the reducing gas is 5%, the flow rate of the reducing gas is 100mL / min, and the catalyst is filled The volume is 12 mL, and the time of the reduction treatment is 8 hours;
[0036] Step 2. Increase the volume content of hydrogen in the r...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com