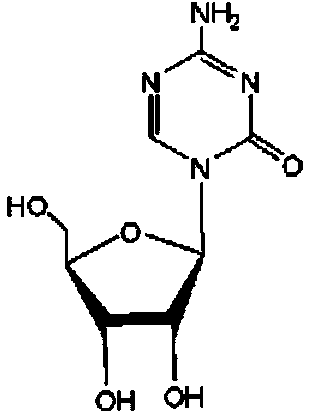
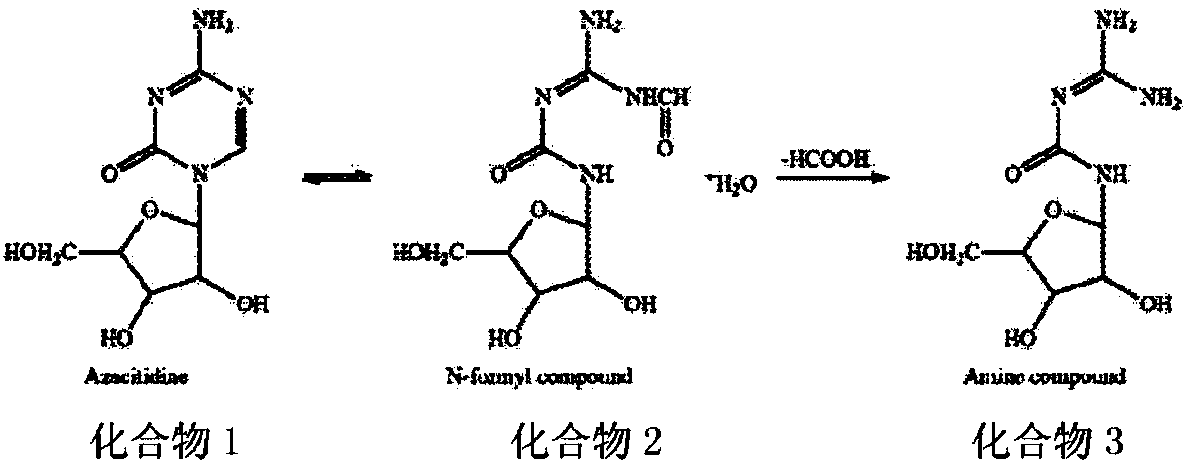
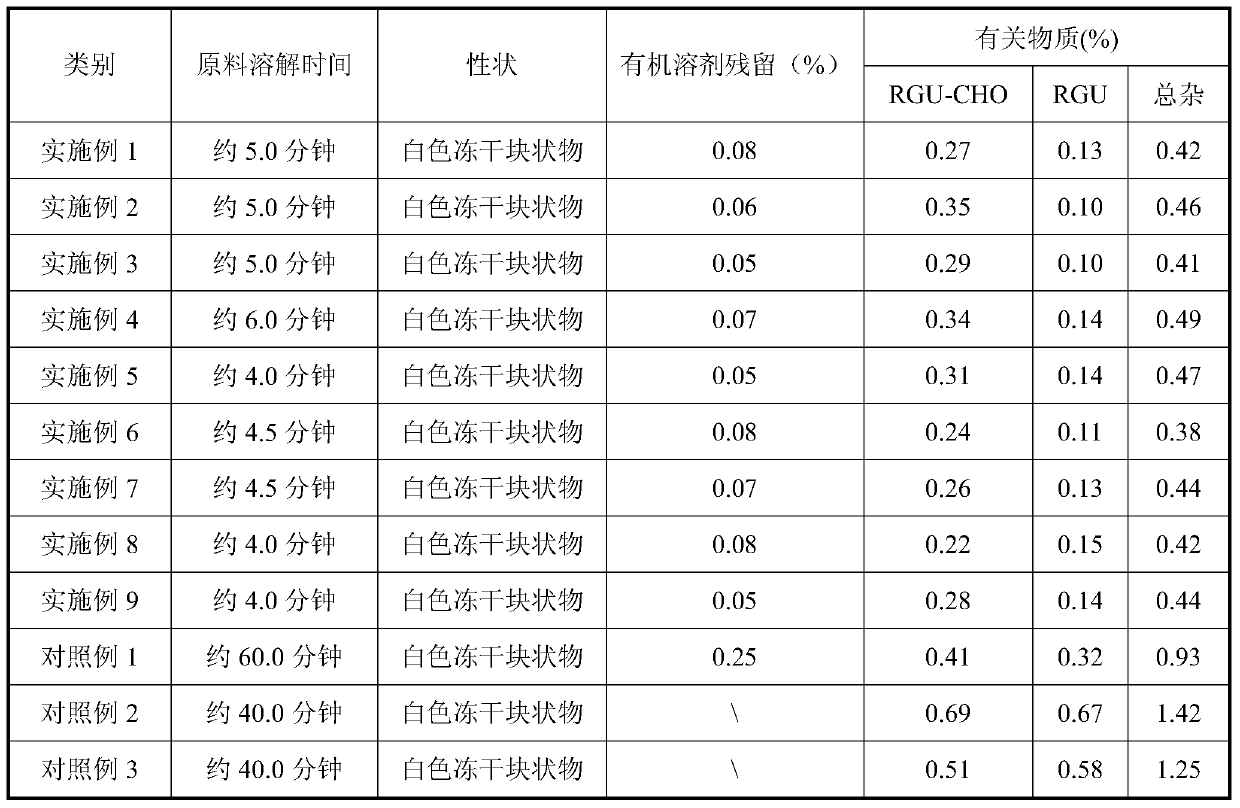
A kind of azacitidine freeze-dried preparation and preparation method thereof
A technology for azacitidine and freeze-dried preparations, applied in the field of azacitidine freeze-dried preparations and preparations, can solve the problems of unsatisfactory, high residual, large amount of organic solvents, etc., and achieve the reduction of product-related substances and physical and chemical stability Good performance and low amount of organic solvent
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039]Measure 950ml of water for injection, cool to -3~-1℃, weigh 5g of mannitol and dissolve it in the above water for injection, weigh 5g of azacitidine, stir and disperse azacitidine into 20ml of ethanol, and dissolve azacitidine After mixing and dissolving the cytidine ethanol dispersion and water for injection, add water to 1000ml, stir the material liquid evenly and filter it with a 0.22μm microporous membrane. The filtered solution is divided into glass bottles, 20ml / bottle, and freeze-dried to obtain azacitidine freeze-dried powder.
Embodiment 2
[0041] Measure 950ml of water for injection, cool to -3~-1℃, weigh 5g of mannitol and dissolve it in the above water for injection, weigh 5g of azacitidine, stir and disperse azacitidine into 20ml of isopropanol, After mixing and dissolving the isopropanol dispersion of azacitidine and water for injection, add water to 1000ml, stir the material evenly and filter it with a 0.22μm microporous membrane. The filtered solution is divided into glass bottles, 20ml / bottle, and freeze-dried Azacitidine freeze-dried powder was obtained.
Embodiment 3
[0043] Measure 950ml of water for injection, cool to -3~-1°C, weigh 5g of mannitol and dissolve it in the above water for injection, weigh 5g of azacitidine, stir and disperse azacitidine into 20ml of methanol, and dissolve azacitidine After the methanol dispersion of cytidine and water for injection are mixed and dissolved, add water to 1000ml, stir the material liquid evenly, filter it with a 0.22μm microporous membrane, pack the filtered solution into glass bottles, 20ml / bottle, and freeze-dry to obtain Azacella Glycoside freeze-dried powder.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com