High temperature xylanase gene and high temperature alpha-glucuronidase gene, and protein expression methods and applications thereof
A technology of xylanase and aldolidase, which is applied in the fields of genetic engineering and biomass utilization, can solve the problems of poor stability, low yield, and low activity of xylanase preparations, and achieve good thermal stability and optimum The effect of high reaction temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
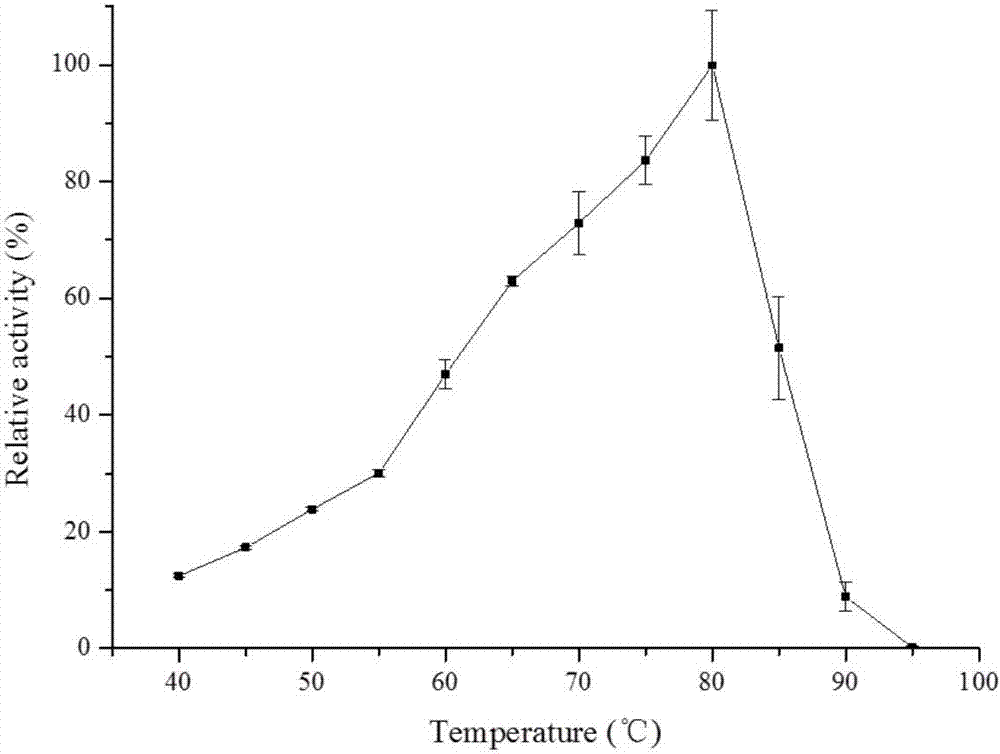
Examples
Embodiment 1
[0031] Example 1: Construction of high temperature xylanase gene Xyn10A and α-glucuronidase gene Agu67A engineering bacteria
[0032] 1.1 Genomic DNA extraction
[0033] The bacterial genome of Caldicellulosiruptorlactoaceticus 6A was extracted with a bacterial genomic DNA extraction kit to obtain genomic DNA, which was frozen at -20°C for future use.
[0034] 1.2 Primer design
[0035] According to the published C.lactoaceticus 6A genome information, the xylanase and α-glucuronidase genes were predicted, and the following 2 pairs of primers were designed:
[0036] The primers used to amplify the xylanase gene are as follows, and the two ends of the primers are respectively introduced with Nhe I and Hind III restriction sites:
[0037] Calla_1331-F 5'-CTA GCTAGC ATGGCTAATTATGAGCATC-3'
[0038] Calla_1331-R 5'-CCC AAGCTT TTAAAGAGTAATTTCAATAAACTTG-3'
[0039] The primers used to amplify the α-glucuronidase gene are as follows:
[0040] Calla_1259-F 5'-GCCGCGCGGCAGCATGAT...
Embodiment 2
[0054] Example 2: Induced expression of recombinant xylanase Xyn10A and α-glucuronidase Agu67A
[0055] Recombinant bacteria were inoculated in 5ml LB liquid medium containing 50μg / ml kanamycin at 1% inoculum size, and cultured overnight at 37°C with shaking at 200rpm. Transfer to 100ml LB liquid medium containing 50μg / ml kanamycin according to the same inoculum amount, shake culture at 200rpm at 37°C until OD 600 When it reaches about 0.4, take out the culture medium and bathe in ice for 10 minutes. Add IPTG to a final concentration of 0.1 mM, shake at 200 rpm at 16°C overnight. The next day, centrifuge at 4000g for 15min, add 3ml Binding Buffer (50mM Tris-HCl pH7.5, 300mMNaCl) to resuspend and collect the bacteria. After sonication, the supernatant obtained by centrifugation at 10,000g at 4°C for 15 minutes was the crude enzyme solution.
Embodiment 3
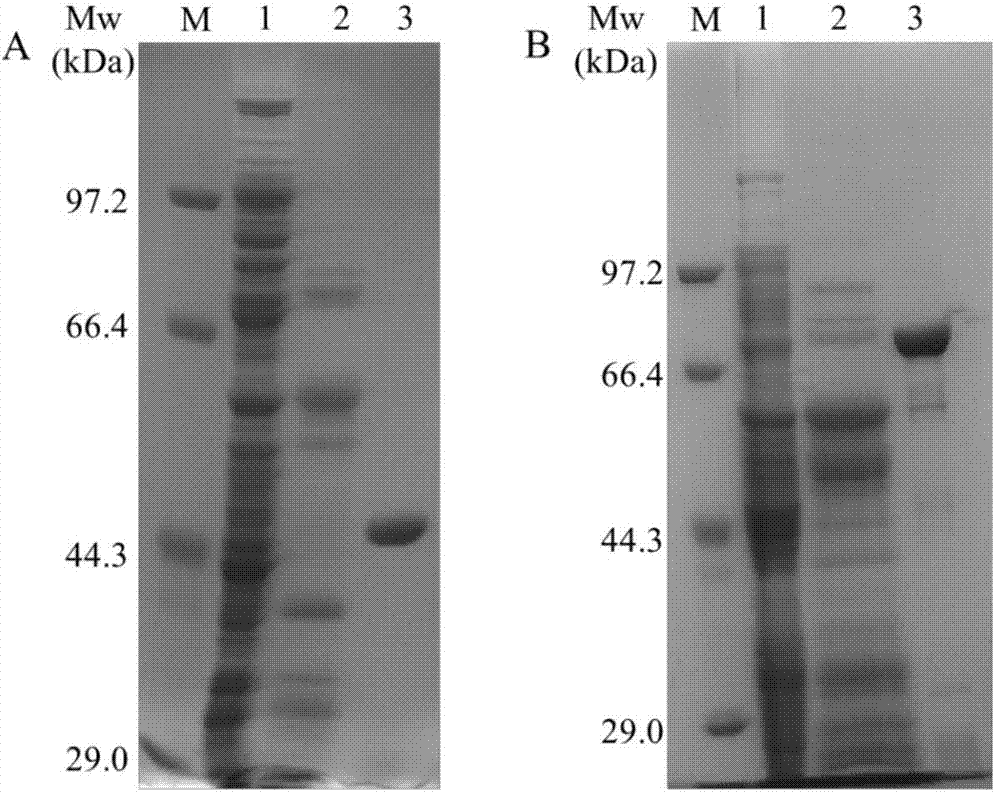
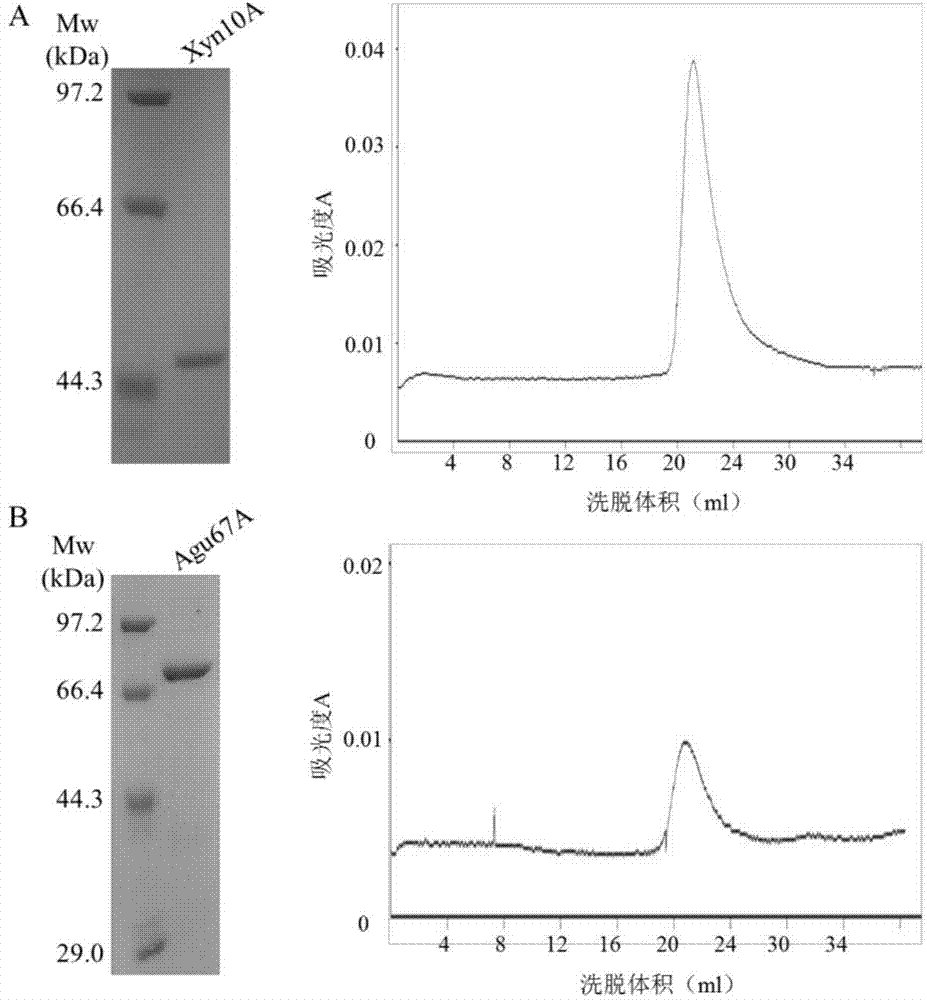
[0056] Example 3: Purification of recombinant xylanase Xyn10A and α-glucuronidase Agu67A
[0057] Using recombinant enzyme C, the N-terminus contains His-Tag, the recombinant protein was separated by heat inactivation at 65°C and Ni-NAT affinity chromatography. The crude enzyme solution was heat-treated at 65°C for 30 minutes to remove heat-labile proteins, and centrifuged at 10,000 g at 4°C for 15 minutes to obtain the supernatant. After 600μl Ni-NAT affinity chromatography column was equilibrated with 6ml Binding Buffer, the supernatant was passed through the column. After passing through the column, wash with 6ml Binding Buffer, then elute with 400μl Elution Buffer (50mM Tris-HCl pH7.5, 300mMNaCl, 150mM imidazole), and collect the eluted sample solution. A 30ml Superdex200 Sephadex column (GE Healthcare) was pre-equilibrated with citric acid buffer (50mM citrate pH6.0, 150mM NaCl), and the eluted sample solution was passed through the column, and the protein was collected ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com