Anionic antibacterial peptide AcHy-a2 in bee venom as well as preparation method and application of anionic antibacterial peptide AcHy-a2
A technology of achy-a2 and antimicrobial peptides, applied in the field of biomedicine, can solve the problems of limiting the utilization of antibacterial properties, and achieve the effects of less drug resistance, simple structure, and less toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Embodiment 1 Preparation of honeybee venom polypeptide
[0040] 1. Honeybee Venom Extraction and Reductive Alkylation
[0041] The abdomen of 10 bees of the genus Apiscerana (Apiscerana) was dissected to extract the venom glands, and the venom was lysed with 8M urea (containing protease inhibitors). The protein concentration in the venom was determined to be 7.87mg / ml by Bradford method. The total protein amount was taken as 1mg, and DTT with a final concentration of 10mM was added, and reacted at 56°C for 1h. After reduction and cooling to room temperature, IAM with a final concentration of 55mM was added and reacted in a dark room at room temperature for 45min.
[0042] 2. Enrichment of venom peptides
[0043] The honeybee polypeptides treated above were used to enrich the polypeptides in the honeybee venom with a Strata-X C18 column. Strata-X C18 enrichment operation was performed according to standard procedures: 1) Add 1ml of methanol to activate the column; 2) ...
Embodiment 2
[0044] Embodiment 2 Identification of anionic antimicrobial peptide AcHy-a2 of the present invention
[0045] 1. Identification of honey bee polypeptide sequence
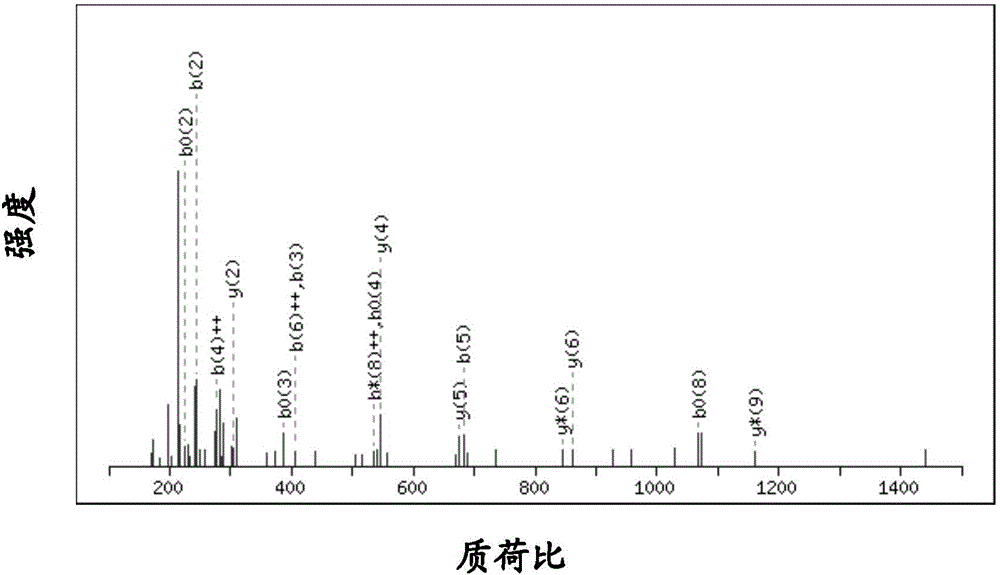
[0046] The enriched polypeptide obtained in Example 1 was lyophilized and reconstituted with 15 μl of 0.1% formic acid, and then analyzed by nanoLC-MS / MS.
[0047] 2. NanoLC-MS / MS analysis
[0048] The liquid-mass spectrometer adopts the nano HPLC chromatographic system of Shimadzu, Japan and the Q-Exactive mass spectrometer system of Thermo Fisher Scientific. The peptide liquid reconstituted in the previous step was separated by the built-in 12cm long, 75μm inner diameter, and Ultimate capillary analysis column filled with Welch Materials brand XB-C18 column material with a particle size of 3μm and a pore size of 120A, with a flow rate of 300nl / min. The detection injection volume is 12 μl, and the elution gradient is such that the B solution concentration rises uniformly from 5% to 30% over 40 minutes. The mass ...
Embodiment 3
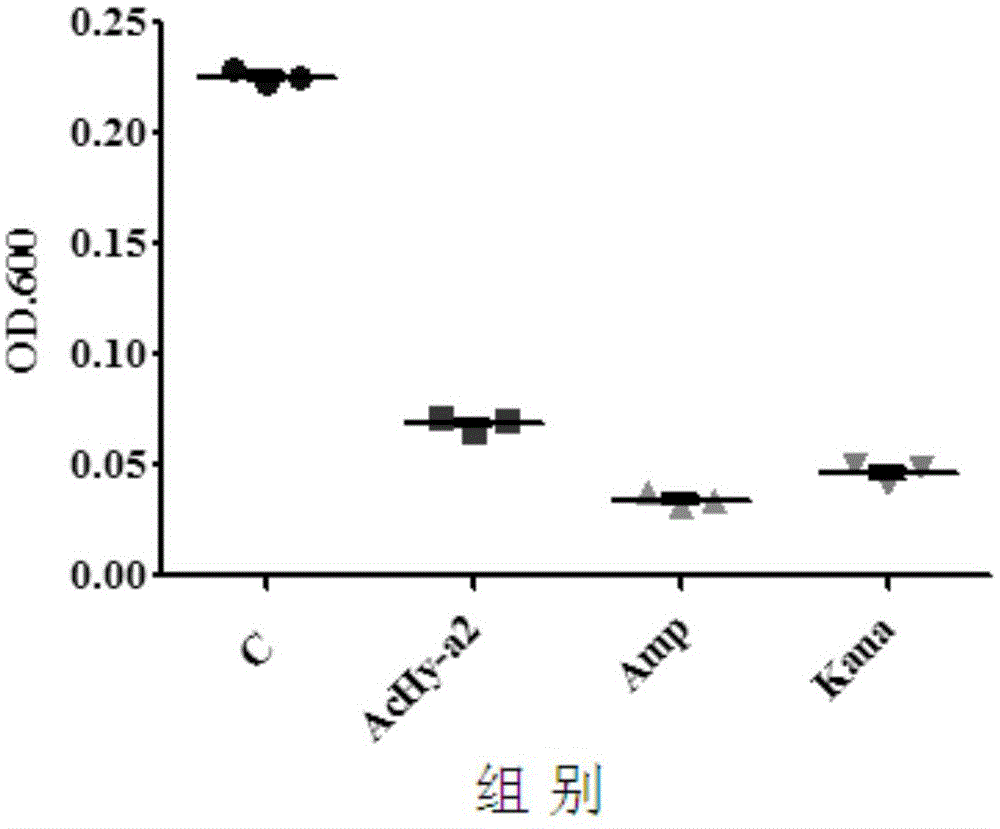
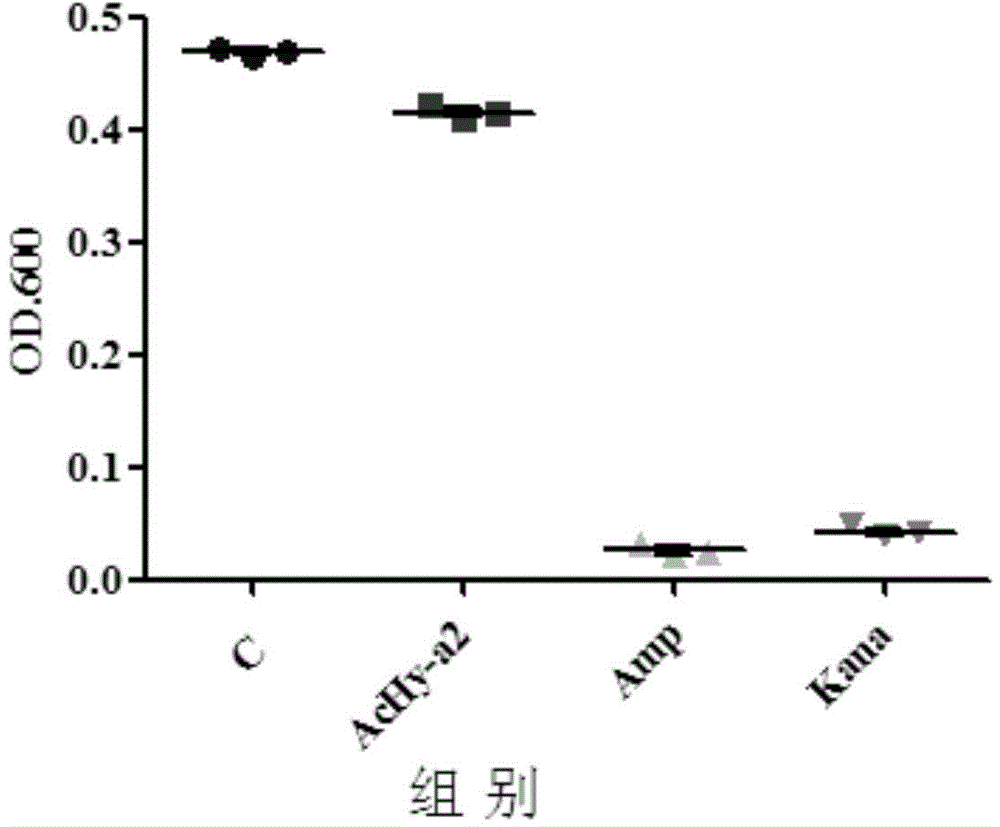
[0053] Example 3 Bacteriostatic activity detection of bee venom anionic antimicrobial peptide AcHy-a2 of the present invention
[0054] The bee venom anionic antibacterial peptide AcHy-a2 synthesized in Example 2 was weighed, and the bactericidal activity of the antimicrobial peptide AcHy-a2 was detected by the microdilution method, and the broad-spectrum semi-synthetic antibiotic Amp and the aminoglycoside antibiotic Kana were used as positive controls , to evaluate the antibacterial activity of the antimicrobial peptide AcHy-a2 of the present invention.
[0055] The test steps are as follows: strain recovery, the standard strain Staphylococcus aureus [CMCC(B)26003-5a20] was inoculated in the nutrient broth medium, cultivated in a biochemical incubator at 37°C for 24 hours, and then purified by streaking on LB solid medium After culturing, take an inoculation loop and inoculate in nutrient broth medium, incubate at a constant temperature of 37°C for 6 hours, count with a bact...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com