Medical lansoprazole composition for treating gastric ulcer
A technology of lansoprazole and composition, which is applied in the field of lansoprazole enteric-coated tablet composition, can solve problems such as unsatisfactory results and influence on drug quality, achieve small content changes, improve dissolution rate, and good fluidity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1: Preparation of Lansoprazole Crystals
[0044] Get lansoprazole crude drug, add 15-25 ℃ volume in the mixed solvent of methanol, dimethylformamide, carbon tetrachloride that is 8 times of the weight of lansoprazole, methanol, dimethylformamide, tetrachloride Carbon chloride volume ratio is 3:2.5:1, obtains solution; Apply the constant magnetic field that magnetic field intensity is 0.5T-0.8T then on the horizontal direction of the liquid surface of gained solution, and under the condition of this constant magnetic field, to solution Dropping volume is lansoprazole weight 10 times the mixed solvent of acetone, ethyl acetate, ether, the volume ratio of acetone, ethyl acetate and ether is 5:3:2.5; , washed, and vacuum-dried for 3 hours to obtain the lansoprazole compound.
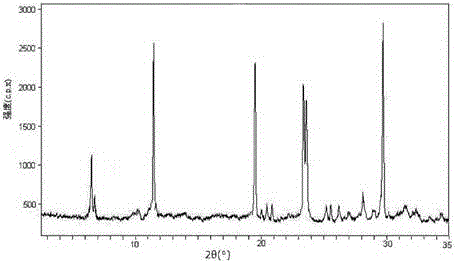
[0045] The X-ray powder diffraction pattern that the prepared lansoprazole crystal uses Cu-Kα ray measurement to obtain is as follows figure 1 Shown, its purity as determined by high perfo...
Embodiment 2
[0046] Example 2: The preparation of Lansol enteric-coated tablets, the steps are as follows:
[0047] Prescription: in parts by weight
[0048]
[0049] Preparation:
[0050] 1) Processing of raw and auxiliary materials: use a vibrating sieve powder machine to sieve the raw material lansoprazole to 80 mesh, and use a pulverizer to crush calcium hydrogen phosphate to pass through a 120 mesh sieve;
[0051] 2) Weigh the raw and auxiliary materials according to the prescription quantity;
[0052] 3) Preparation of adhesive: Take purified water at 70-80°C and place it in a stainless steel bucket, add hypromellose while stirring, stir until completely dissolved, wait until the temperature drops to room temperature, add sodium lauryl sulfate, and stir 4) Mix and granulate: add lansoprazole crystals, lactose, microcrystalline cellulose, carboxymethyl starch sodium, and calcium hydrogen phosphate into the wet mixing granulator, turn on the stirring motor and dry mix for 10 min...
Embodiment 3
[0058] Example 3: The preparation of Lansol enteric-coated tablets, the steps are as follows:
[0059] Prescription: in parts by weight
[0060]
[0061] Preparation:
[0062] 1) Processing of raw and auxiliary materials: use a vibrating sieve powder machine to sieve the raw material lansoprazole to 80 mesh, and use a pulverizer to crush calcium hydrogen phosphate to pass through a 120 mesh sieve;
[0063] 2) Weigh the raw and auxiliary materials according to the prescription quantity;
[0064] 3) Preparation of adhesive: Take purified water at 70-80°C and place it in a stainless steel bucket, add hypromellose while stirring, stir until completely dissolved, wait until the temperature drops to room temperature, add sodium lauryl sulfate, and stir 4) Mix and granulate: add lansoprazole crystals, lactose, microcrystalline cellulose, carboxymethyl starch sodium, and calcium hydrogen phosphate into the wet mixing granulator, turn on the stirring motor and dry mix for 10 min...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com