Preparing method of calixarene modified magnetic material
A technology of calixarene and magnetic zeolite is applied in the field of preparation of magnetic materials, which can solve the problems of complicated operation steps, long time consumption, easy oxidation of ferromagnetic particles, etc., and achieves the advantages of improving enrichment efficiency, stable material performance and excellent application potential. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
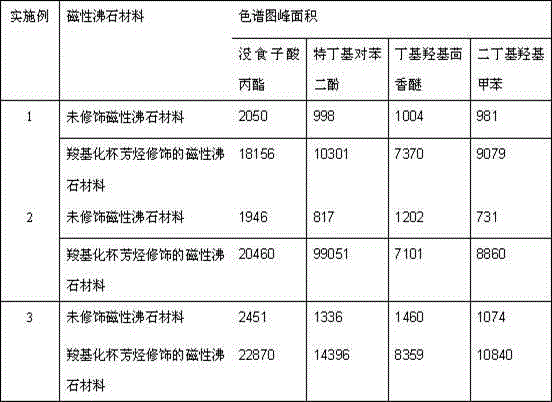
Embodiment 1
[0015] (1) Take 5.0g of mesoporous silica gel and 4.0g of ferric nitrate as reaction monomers, mix and stir them evenly in ethanol solution, and dry at 35°C to powder; add 2mL of ethylene glycol as a reducing agent, and the powder Place in a tube furnace, under the protection of nitrogen, react at 230°C for 5 hours, the in situ reduction method will produce ferric oxide silicon dioxide dark brown powder; with 0.05g sodium hydroxide as alkali source, 3.5g tetrapropyl hydrogen Ammonium oxide was used as a structure-directing agent, 0.05g of sodium metaaluminate was used as a reaction monomer, and 16mL of deionized water was used as a reaction medium. 4.0g of dark brown powder was injected into the reaction kettle and reacted at 160°C for 20 After cooling, magnetic zeolite was obtained;
[0016] (2) With 7.0mL ethanol as the reaction medium, 6.0mL deionized water as the hydrolysis initiator, and 1.0mL 3-aminopropyltriethoxysilane as the silylating agent, the magnetic zeolite is c...
Embodiment 2
[0019] (1) Take 5.0g of mesoporous silica gel and 5.0g of ferric nitrate as reaction monomers, mix and stir them evenly in an ethanol solution, and dry them at 50°C until they are powdery; after adding 5mL of ethylene glycol as a reducing agent, the powder Put it in a tube furnace, under the protection of nitrogen, react at 280°C for 7 hours, the in situ reduction method will generate ferric oxide silicon dioxide dark brown powder; with 0.2g sodium hydroxide as alkali source, 5.0g tetrapropyl hydrogen Ammonium oxide was used as a structure-directing agent, 0.2g of sodium metaaluminate was used as a reaction monomer, and 20mL of deionized water was used as a reaction medium. 4.0g of dark brown powder was injected into the reaction kettle and reacted at 200°C for 30 After cooling, magnetic zeolite was obtained;
[0020] (2) With 10.0mL ethanol as the reaction medium, 7.0mL deionized water as the hydrolysis initiator, and 3.0mL 3-aminopropyltriethoxysilane as the silylating agent...
Embodiment 3
[0023] (1) Take 5.0g of mesoporous silica gel and 4.0g of ferric nitrate as reaction monomers, mix and stir them evenly in an ethanol solution, and dry them at 40°C until they are powdery; after adding 4mL of ethylene glycol as a reducing agent, the powder Placed in a tube furnace, under the protection of nitrogen, reacted at 250°C for 6 hours, and the in-situ reduction method produced iron ferric oxide and silicon dioxide dark brown powder; with 0.1g sodium hydroxide as the alkali source, 4.1g tetrapropyl hydrogen Ammonium oxide was used as a structure-directing agent, 0.1g of sodium metaaluminate was used as a reaction monomer, and 19mL of deionized water was used as a reaction medium. 4.0g of dark brown powder was injected into the reaction kettle and reacted at 180°C for 24 After cooling, magnetic zeolite was obtained;
[0024] (2) With 9.5mL ethanol as the reaction medium, 6.4mL deionized water as the hydrolysis initiator, and 1.5mL 3-aminopropyltriethoxysilane as the si...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com