Preparation method for double-emission waterborne polyurethane capable of emitting fluorescence and phosphorescence synchronously
A water-based polyurethane, dual-emission technology, applied in the field of preparation of functional polyurethane, can solve the problems of mechanics, processability and adhesion performance, easily degradable price, expensive and other problems, and achieve lasting and stable luminous performance, not easy to migrate, and low cost. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
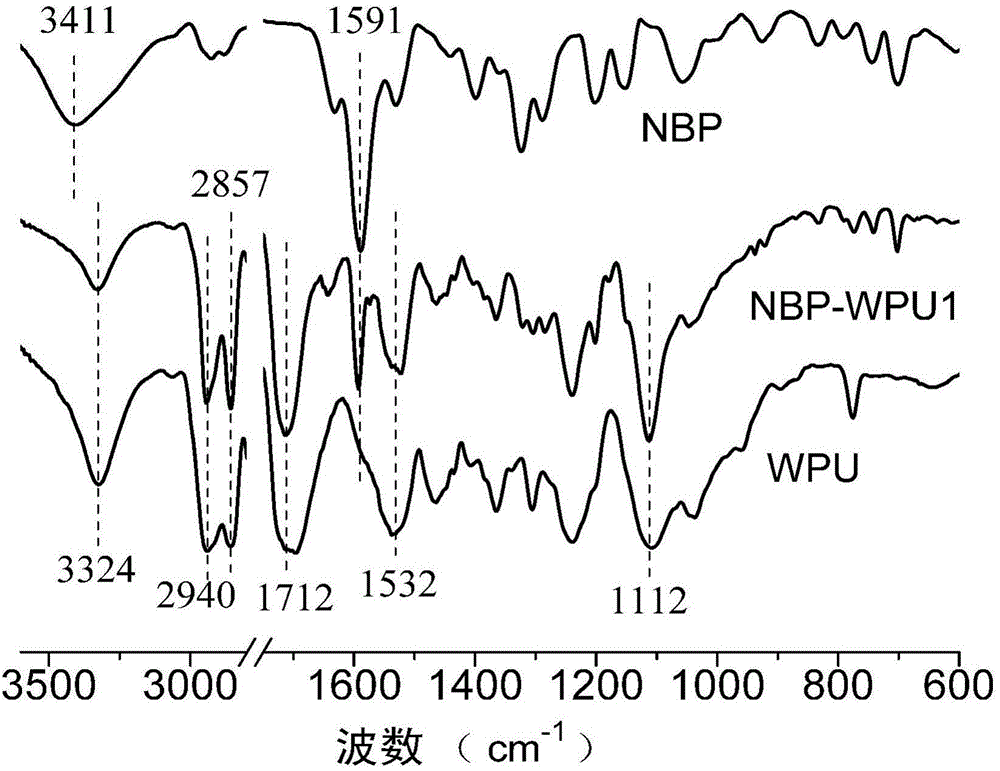
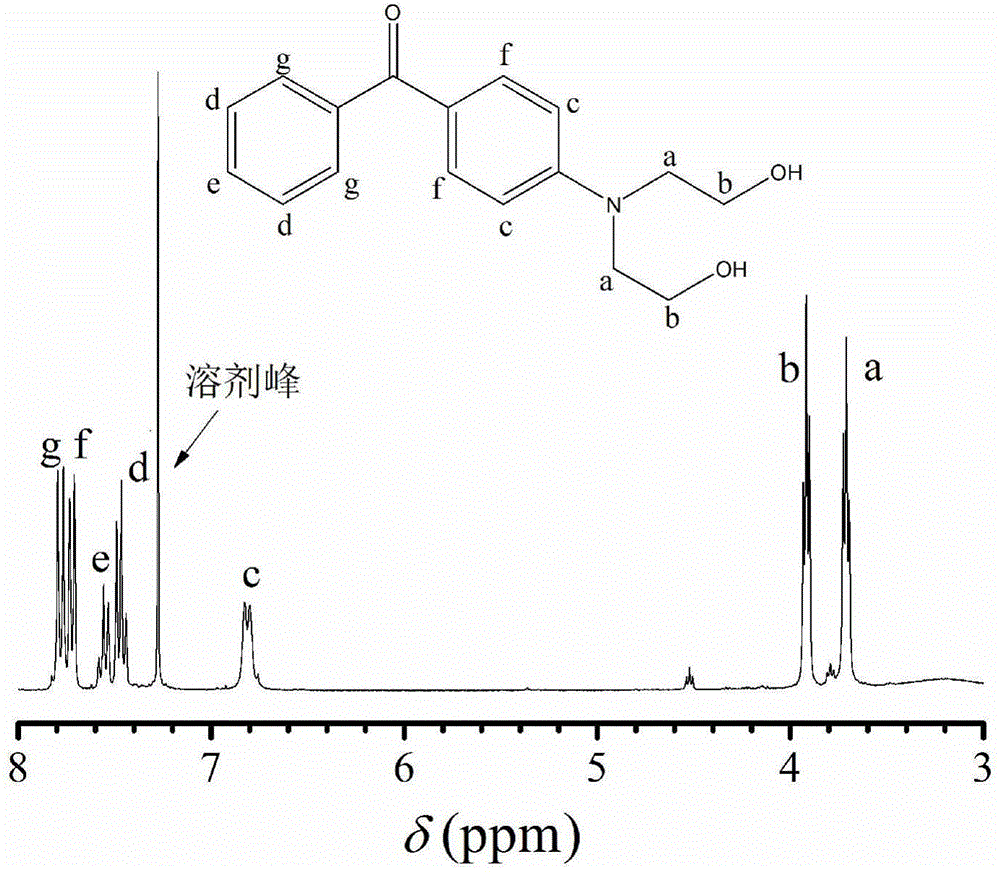
[0041]1. Add 4.32 grams of 4-chlorobenzophenone, 14.32 grams of diethanolamine and 1.46 grams of potassium hydroxide into a 250mL three-necked flask, stir and heat up to 130°C, and finish the reaction after 14 hours of reaction; After cooling to room temperature, add 100mL of distilled water, stir rapidly for 60 minutes, remove the water in the upper layer after the viscous precipitate is separated out; obtain a yellow solution after separation and purification by column chromatography (eluent is ethyl acetate), reduce The solvent was removed by pressure distillation, and after vacuum drying, 2.23 g of brown-yellow fluorescent and phosphorescent dual-emission diols {4-[(N,N-dihydroxyethyl)-amino]-phenyl}-phenyl-methanone were obtained (NBP).
[0042] 2. The number average molecular weight M n PTMG of 2000 was dehydrated at 110°C for 1 hour and then cooled to 50°C. Take 6.46 grams and add it to a 100mL three-necked flask, then add 5 grams of IPDI, stir and raise the temperatur...
Embodiment 2
[0048] The number average molecular weight M n 2000 PTMG was dehydrated at 110°C for 1 hour and then cooled to 50°C. Take 6.46 grams and add it to a 100mL three-neck flask, then add 5 grams of IPDI, stir and heat up to 90°C for 2 hours, then add 0.13 grams of Example 1 The prepared NBP, 0.8 g of DMPA, 0.96 g of BDO, 0.01 g of DBTDL and 10 g of acetone were stirred and reacted at a constant temperature of 70 ° C for 6 hours and then cooled to 20 ° C; Add 30 grams of water under high-speed stirring (3000 rpm), and transfer the reaction product to a rotary evaporator after 30 minutes, and remove acetone at 45°C and 0.01MPa vacuum to obtain anionic water-based polyurethane emulsion NBP-WPU2.
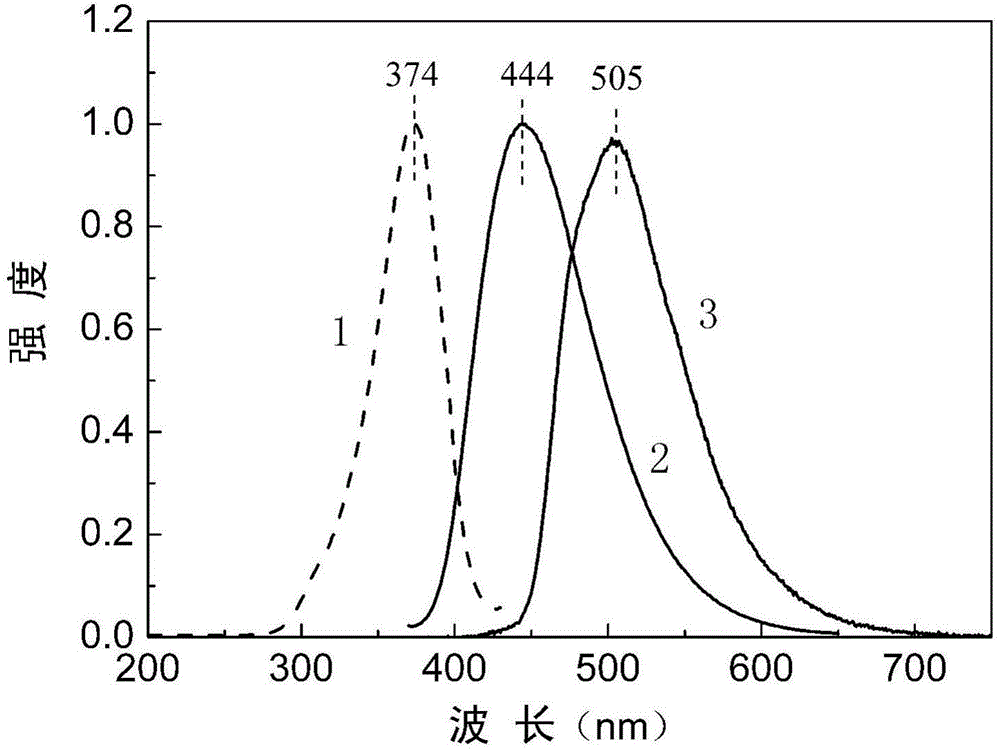
[0049] image 3 The photoluminescence excitation spectrum and photoluminescence emission spectrum of the film formed by the NBP-WPU2 emulsion prepared in this example. Curve 1 is the excitation spectrum with the maximum wavelength at 374nm; curve 2 is the fluorescence emission spectrum wit...
Embodiment 3
[0056] The number average molecular weight M n 2000 PPG was dehydrated at 110°C for 1 hour and then cooled to 50°C. Take 19.38 grams and add it to a 250mL three-neck flask, then add 15 grams of IPDI, stir and heat up to 90°C for 2 hours, then add 0.42 grams of Example 1 The prepared NBP, 1.6 grams of DEG, 0.03 grams of DBTDL and 10.5 grams of methyl ethyl ketone were stirred and reacted at a constant temperature of 75 ° C for 3 hours and then cooled to 45 ° C; then a solution formed by 4.2 grams of MDEA and 4.2 grams of methyl ethyl ketone was added dropwise within 1 hour After reacting for 2 hours at 60°C, the reaction product was transferred to a high-speed shear disperser, and 2.13 grams of acetic acid was added under the shear condition of 3000 rpm; 90 grams of water was added after 5 minutes of reaction, and the reaction was stirred for 1 minute. The product was transferred to a rotary evaporator, and butanone was removed under 50°C and 0.01MPa vacuum conditions to obtain...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com