Photoluminescent room-temperature ionic liquid preparation method
A room temperature ionic liquid and photoluminescence technology, which is applied in luminescent materials, chemical instruments and methods, organic chemistry, etc., can solve the problems of lack of preparation methods, high thermal stability, high melting point, etc., and achieve simple, feasible and thermally stable preparation methods The effect of high resistance and low melting point
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
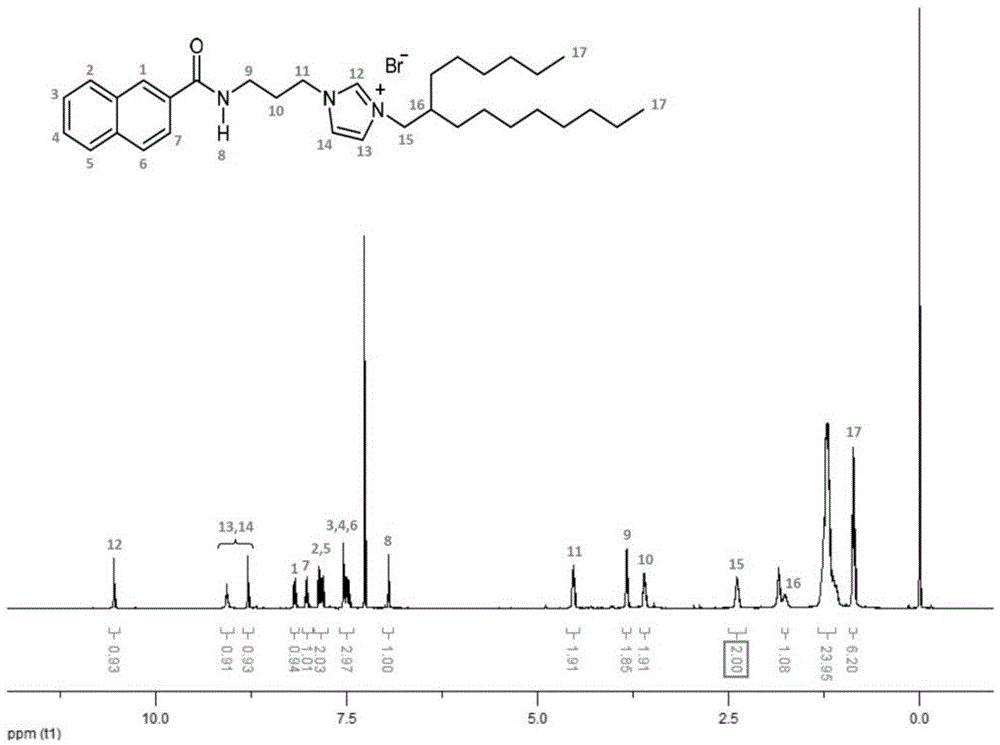
[0046] 1. Add 20 milliliters of thionyl chloride to a round-bottomed flask containing 2.0 grams of 2-naphthoic acid, stir at room temperature for 6 hours, remove thionyl chloride under reduced pressure, add 10 milliliters of dichloromethane, add N-(3- Aminopropyl) imidazole dichloromethane solution (1.4 milliliters dissolved in 15 milliliters of dichloromethane) and 1 mole per liter of sodium hydroxide aqueous solution (10 milliliters), stirred at room temperature for 12 hours, separated to remove the aqueous phase, and collected dichloromethane Methane phase, dichloromethane was removed under reduced pressure, 50 ml of cold water was added, extracted three times with chloroform (20 ml each time), the chloroform phase was combined, dried over anhydrous magnesium sulfate, suction filtered, chloroform was removed under reduced pressure, and CH 3 OH / CH 2 Cl 2 (V CH3OH =15%, containing 0.1% ammonia water) mixed solvent was used as eluent to carry out silica gel column chromatogr...
Embodiment 2
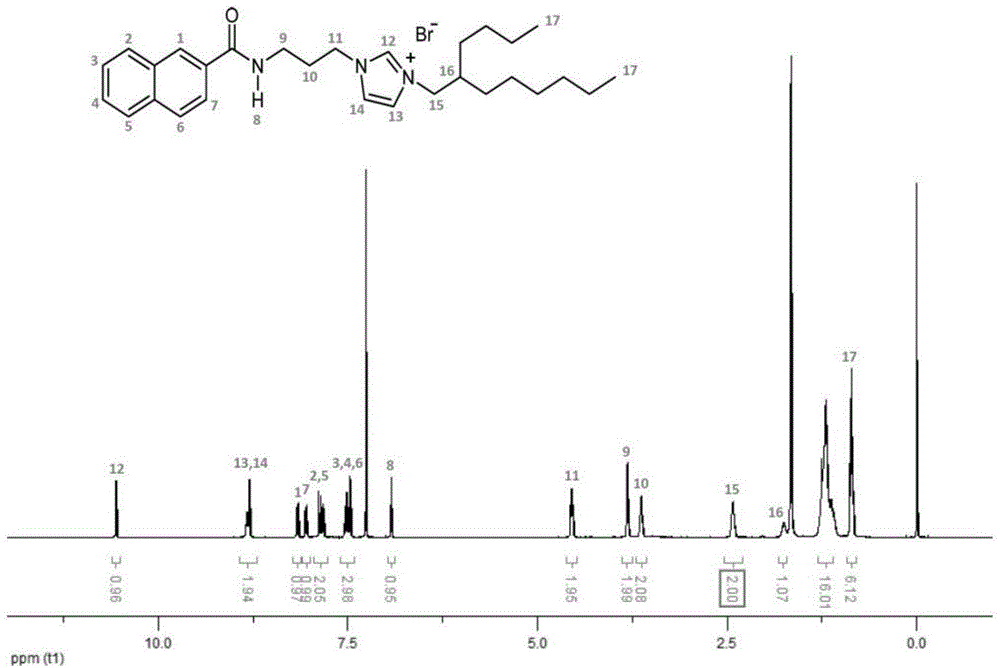
[0051] On the basis of Example 1, the 2-butyl-1-octanol in step 2. is replaced with 2-hexyl-1-decanol, the reaction time in step 3. is adjusted to 4 days, and other conditions are unchanged, to obtain 1-(3-(2-naphthoyl)propyl)-3-(2-hexyldecyl)-1H-imidazole-3-bromide salt, the yield is 38%, its 1H NMR and electrospray mass spectrometry results are shown in figure 2 and Image 6 , and the thermogravimetric analysis results are shown in Figure 9 b, The results of differential scanning calorimetry are shown in Figure 10 b, Rheological measurements are shown in Figure 12-Figure 14 , the photoluminescence behavior in ethanol solution (0.1mol / L) is shown in Figure 17 , the photoluminescence behaviors under solvent-free conditions and on various solid substrates are shown in Figure 18 b and Figure 19 .
Embodiment 3
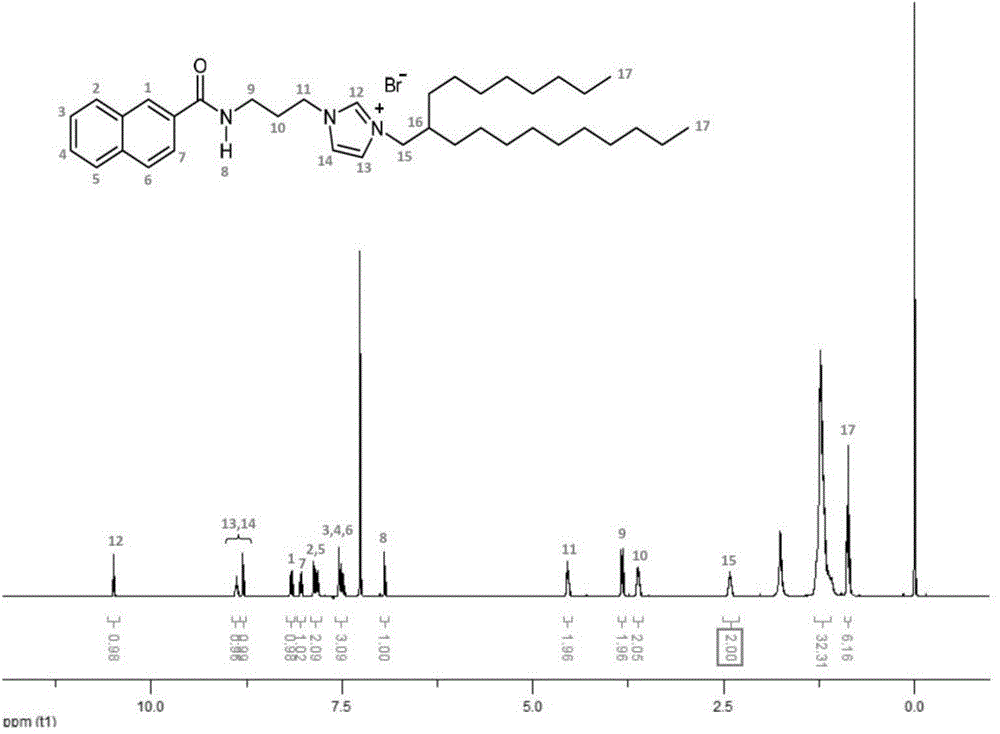
[0053] On the basis of Example 1, the 2-butyl-1-octanol in step ② is replaced with 2-octyl-1-dodecanol, the reaction time in step ③ is adjusted to 5 days, and other conditions remain unchanged , to get 1-(3-(2-naphthoyl)propyl)-3-(2-octyldodecyl)-1H-imidazole-3-bromide salt, the yield was 37%, and its 1H NMR and electrospray mass spectrometry results are shown in image 3 and Figure 7 , and the thermogravimetric analysis results are shown in Figure 9 c, The results of differential scanning calorimetry are shown in Figure 10 c, The rheological measurement results are shown in Figure 12-Figure 14 , the fluid properties at room temperature are shown in Figure 11 , the photoluminescence behavior under solvent-free conditions is shown in Figure 18 c, d, e.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thermal decomposition temperature | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com