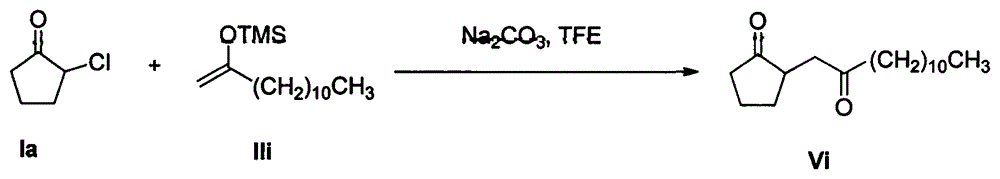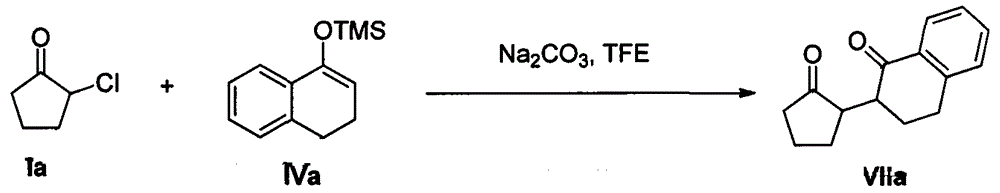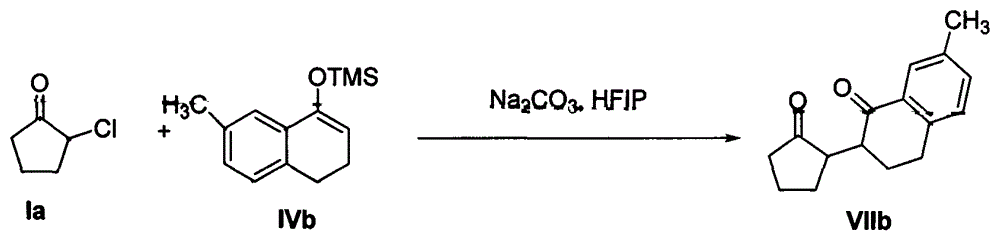Method for synthesizing 1,4-diketone compound by using 2-halogenated cyclopentanone as raw material
A technology for substituting cyclopentanone and compounds, applied in the field of synthesis 1, which can solve the problems of harsh reaction conditions, limited range of substrates, and difficulty in obtaining raw materials, and achieve the effects of less side reactions, low cost, and easy-to-obtain raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1: Reaction of 2-chlorocyclopentanone Ia with enol silyl ether IIa
[0025]
[0026] In a 50mL round-bottom flask, under stirring, add sodium carbonate (15mmol), 2-chlorocyclopentanone Ia (10mmol) and 15mL trifluoroethanol solvent, then enol silyl ether IIa (30mmol) by constant pressure dropwise The funnel drips slowly into the round bottom flask. The reaction mixture was stirred at room temperature for 12 h. After the reaction of 2-chlorocyclopentanone Ia was monitored by TCL, the trifluoroethanol solvent was recovered by distillation under reduced pressure, and the remaining mixture was added with 15 mL of water and 15 mL of dichloromethane and stirred at room temperature for 0.5 h, and then extracted with dichloromethane. The extract was dried with anhydrous sodium sulfate and concentrated; then the mixed solvent of petroleum ether and ethyl acetate (10:1 by volume) was used as the eluent to carry out separation and purification by silica gel column chrom...
Embodiment 2
[0028] Example 2: Reaction of 2-chlorocyclopentanone Ia with enol silyl ether IIb
[0029]
[0030] In a 50mL round-bottomed flask, add 2-chlorocyclopentanone Ia (10mmol), sodium carbonate (15mmol), and 15mL trifluoroethanol solvent under stirring, then pass enol silyl ether IIb (30mmol) through a constant pressure dropping funnel Slowly drop into a round bottom flask. The reaction mixture was stirred at room temperature for 12 h. After TCL monitored the complete reaction of 2-chlorocyclopentanone Ia, the trifluoroethanol solvent was recovered by distillation under reduced pressure, and the remaining mixture was added with 15 mL of water and 15 mL of difluoromethane and stirred at room temperature for 0.5 h, then extracted with difluoromethane. The extract was dried with anhydrous sodium sulfate and concentrated; then the mixed solvent of petroleum ether and ethyl acetate (volume ratio: 10:1) was used as the eluent to carry out silica gel column chromatography for separation...
Embodiment 3
[0032] Example 3: Reaction of 2-chlorocyclopentanone Ia with enol silyl ether IIc
[0033] In a 50mL round-bottomed flask, under stirring, add 2-chlorocyclopentanone Ia (10mmol), potassium carbonate (20mmol) and 10mL hexafluoroisopropanol, then add enol silyl ether IIc (50mmol) in hexafluoroisopropanol The alcohol solution was slowly dropped into the round bottom flask through the constant pressure dropping funnel. The reaction mixture was stirred at 80° C., and after the reaction of 2-chlorocyclopentanone Ia was monitored by TCL, the hexafluoroisopropanol solvent was recovered by distillation under reduced pressure, and the remaining mixture was added with 15 mL of water and 15 mL of dichloromethane and stirred at room temperature for 1 h, and then Extract with dichloromethane. The extract was dried with anhydrous sodium sulfate and concentrated; then the mixed solvent of petroleum ether and ethyl acetate (volume ratio: 10:1) was used as the eluent to carry out silica gel ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com