Preparation method of aminomethylbenzoic acid
A technology of aminotoluene and toluene, which is applied in the field of preparation of aminotoluene, can solve the problems of product crystal structure differences, isopropyltoluene is not easy to obtain, and the steps of the synthesis route are long, etc., and achieves improved product purity, cheap raw materials, and easy operation. easy effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
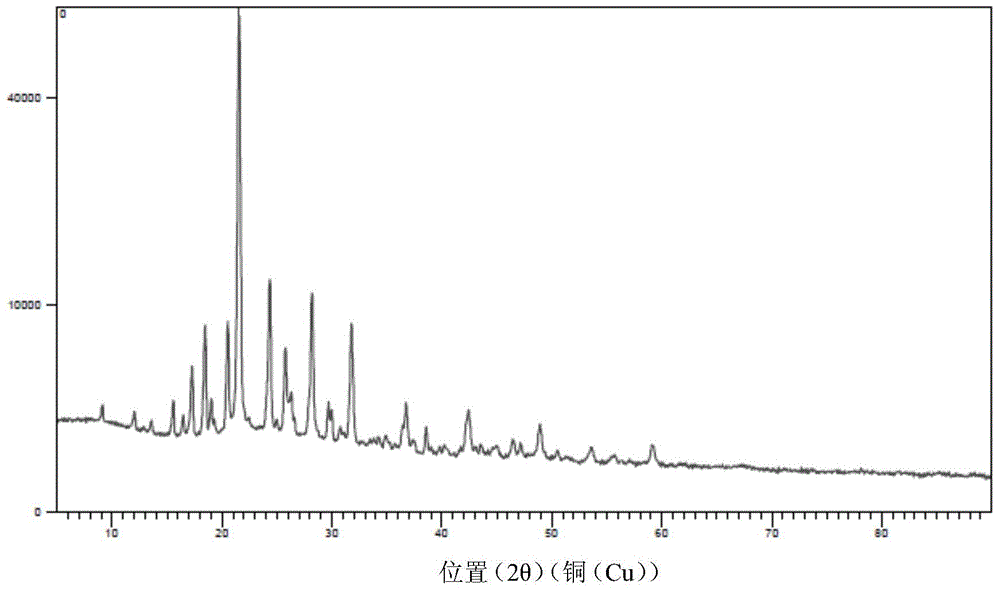
[0053] Embodiment one: the preparation of aminotoluic acid.
[0054] 1. Ammonolysis of cyanobenzyl chloride:
[0055] React according to the formula recorded in the following synthetic route and table:
[0056] synthetic route:
[0057]
[0058] formula:
[0059]
[0060] operate:
[0061] Add toluene (300mL) and p-cyanobenzyl chloride (60g, 0.396mol) into a 500mL four-necked bottle, stir at room temperature to dissolve, and obtain a p-cyanobenzyl chloride solution for later use; product (30g, 0.214mol) and purified water (270mL), stirred at room temperature to dissolve, cooled to 5°C in an ice-salt bath, added 28% ammonia (135g, 2.223mol), and then began to drop p-cyanobenzyl toluene chloride During the dropwise addition, the internal temperature was controlled not to exceed 15°C. After the dropwise addition, the ice-salt bath was removed, and the internal temperature was kept at 15-30°C for 3 hours, heated to 70-75°C in a water bath, kept for 3 hours, and analyzed ...
Embodiment 2
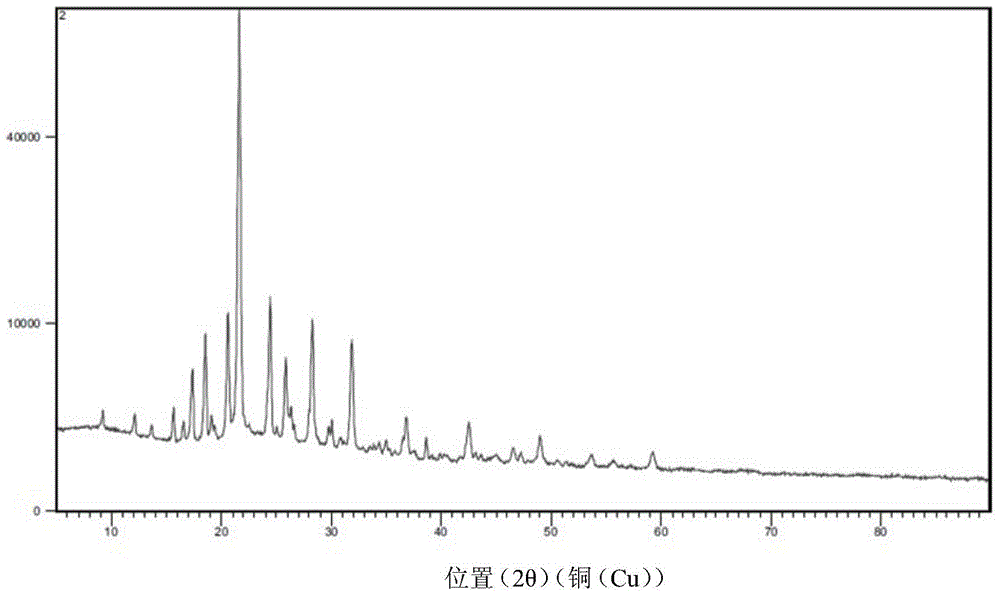
[0075] Embodiment two: the preparation of aminotoluic acid.
[0076] Replace toluene with xylene as solvent, take urotropine as catalyst, operate as above ammonolysis reaction, and carry out ammoniated mother liquor to apply mechanically, the situation is the same as the experimental result of toluene as solvent.
Embodiment 3
[0077] Embodiment three: the preparation of aminotoluic acid.
[0078] Replace toluene with chlorobenzene as solvent, take urotropine as catalyst, operate as above ammonolysis reaction, the result is identical with embodiment one and embodiment two.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com