Preparation method of lithium manganese phosphate and lithium manganese phosphate/carbon composite material
A technology of lithium manganese phosphate and phosphoric acid is applied in the field of preparation of positive electrode materials for lithium ion batteries to achieve the effects of perfect crystal form, good electrochemical performance and short stroke.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
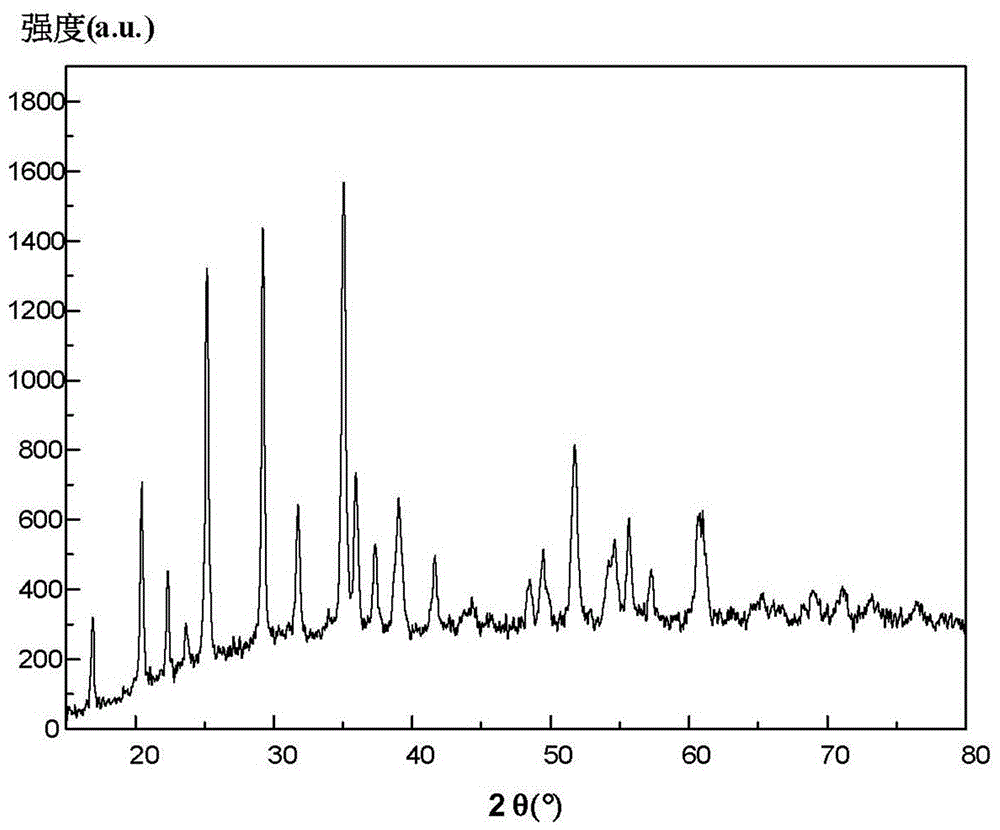
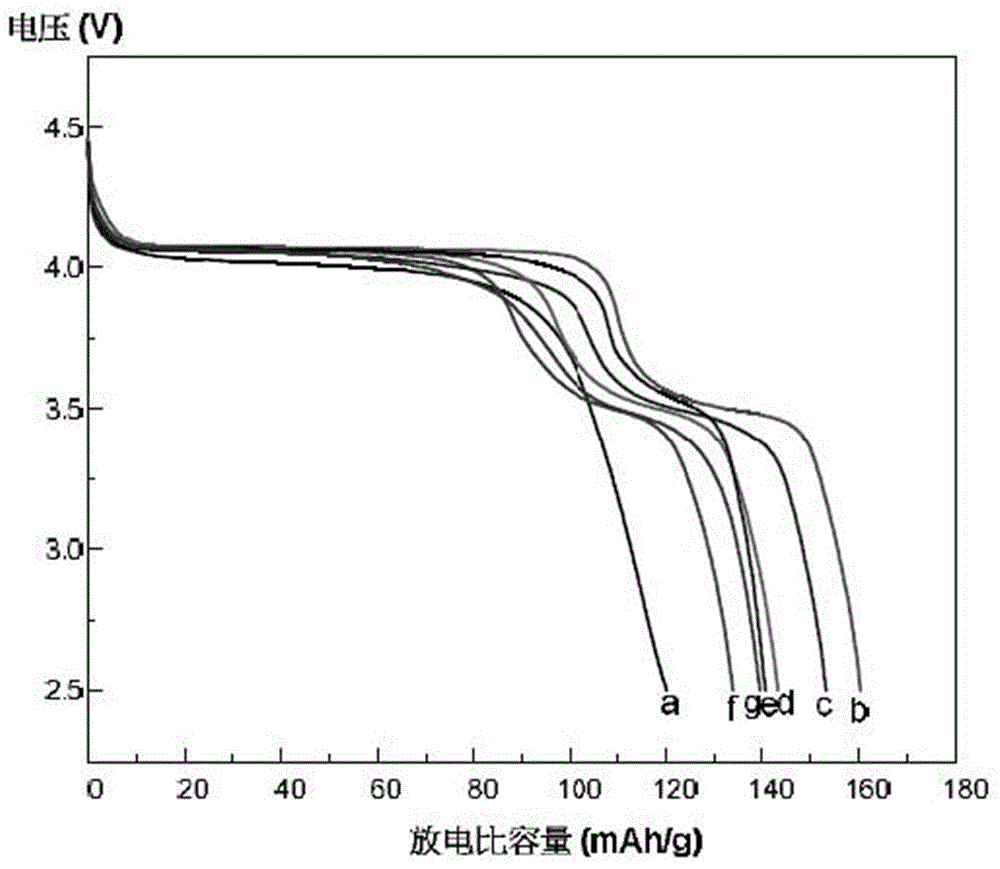
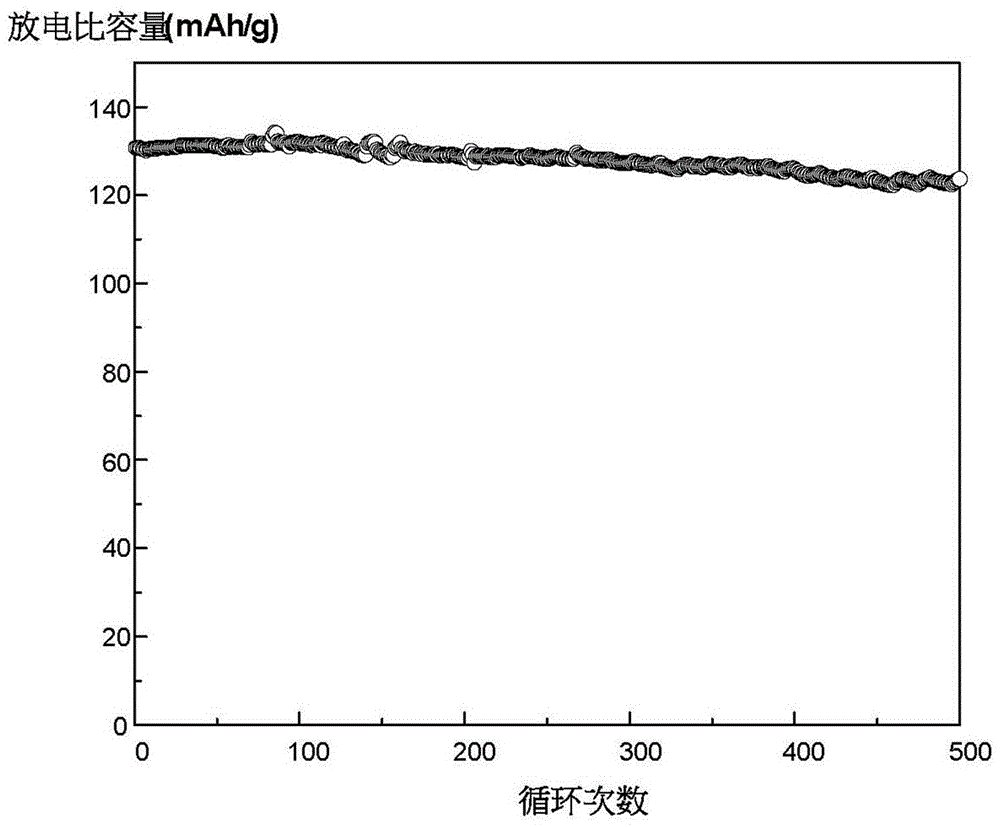
Image
Examples
preparation example Construction
[0013] The embodiment of the present invention provides a preparation method of lithium manganese phosphate, comprising the following steps:
[0014] S1, divalent manganese (Mn 2+ ) source, lithium (Li + ) source and phosphate (PO 4 3+ ) sources are mixed and dissolved in a solvothermal reaction medium to form a mixed solution, and the solvothermal reaction medium includes an organic solvent and a co-solvent;
[0015] S2, performing a solvothermal reaction on the mixed solution to obtain a reaction product lithium manganese phosphate.
[0016] The divalent manganese source may be one or more of manganese chloride, manganese nitrate, manganese sulfate and manganese acetate.
[0017] The lithium source can be one or more of lithium hydroxide, lithium acetate, lithium carbonate and lithium oxalate.
[0018] The phosphate source can be one or more of phosphoric acid, lithium dihydrogen phosphate, ammonium phosphate, diammonium hydrogen phosphate and ammonium dihydrogen phosph...
Embodiment 1
[0051] Measure 70mL of ethylene glycol and 30mL of alkylphenol polyoxyethylene ether and mix evenly, then add 7.916g of manganous chloride tetrahydrate, and stir mechanically for 60 minutes to form a uniform manganous chloride solution. Measure 3mL of phosphoric acid, drop by drop into the manganous chloride solution, and mechanically stir for 2 hours to form a uniform mixed solution A. Then weigh 5.035g of lithium hydroxide monohydrate, add it into 100mL of ethylene glycol, and mechanically stir for 60 minutes to form a uniform lithium hydroxide solution. The solution A was added dropwise into the lithium hydroxide solution, stirred and reacted for 60 minutes, sealed into a high-temperature reactor with a polytetrafluoroethylene liner, kept at a constant temperature of 180°C, and reacted for 5 hours. The obtained product is the lithium manganese phosphate material after being centrifuged, washed and dried. The lithium manganese phosphate material was mixed with 15wt% sucrose...
Embodiment 2
[0054] Measure 70mL of ethylene glycol and 30mL of alkylphenol polyoxyethylene ether and mix evenly, then add 5.533g of manganous chloride tetrahydrate and 3.3362 g of ferrous sulfate heptahydrate, and stir mechanically for 60 minutes to form a uniform mixture of manganous chloride and sulfurous acid iron mixed solution. Measure 3mL of phosphoric acid, drop by drop into the mixed solution of manganous chloride and ferrous sulfate, mechanically stir for 2 hours to form a uniform mixed solution A. Then weigh 5.035g of lithium hydroxide monohydrate, add it into 100mL of ethylene glycol, and mechanically stir for 60 minutes to form a uniform lithium hydroxide solution. The solution A was added dropwise into the lithium hydroxide solution, stirred and reacted for 60 minutes, sealed into a high-temperature reactor with a polytetrafluoroethylene liner, kept at a constant temperature of 180°C, and reacted for 5 hours. The obtained product is centrifuged, washed and dried to be lithiu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| crystal size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com