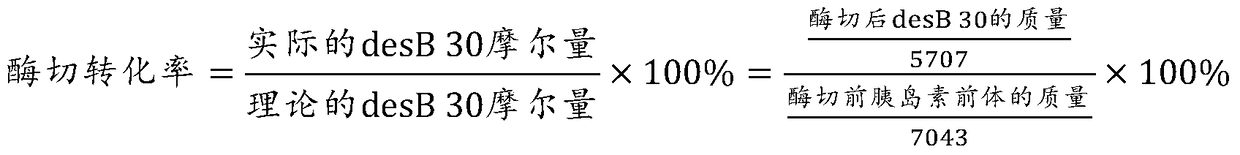Purification and enzymatic conversion method of recombinant human insulin precursor
A technology of recombinant human insulin and conversion method, which is applied in the field of preparation of recombinant human insulin, can solve the problems of complicated operation, reduced sample yield in process steps, complicated sample processing procedures, etc., and achieves the effects of improving purity yield and simplifying purification steps.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Preliminary Example 1 Preparation of Fermentation Supernatant Containing Recombinant Human Insulin Precursor
[0037] Refer to "Process Research on Transformation of Recombinant Insulin Precursor into Human Insulin and Insulin Detemir", Liu Haifeng, East China University of Science and Technology, PhD thesis (2013).
[0038] The artificially synthesized ILP gene and plasmid pPIC9K were digested with Xho I and EcoRI respectively, and the corresponding target fragment and large plasmid fragment were recovered. The two fragments were connected with T4DNA ligase to construct the recombinant plasmid pPIC9K::ILP, and then the constructed A large amount of recombinant plasmid pPIC9K::ILP was prepared, linearized, electroporated and transformed into host cell P.pastorisGS115, coated with MGY (His-) plate, and high-copy positive clones were selected (wherein, the amino acid of the constructed insulin precursor protein (ILP) The sequence (SEQ ID NO.1) is: eeaeaeaepk (spacer pepti...
Embodiment 2
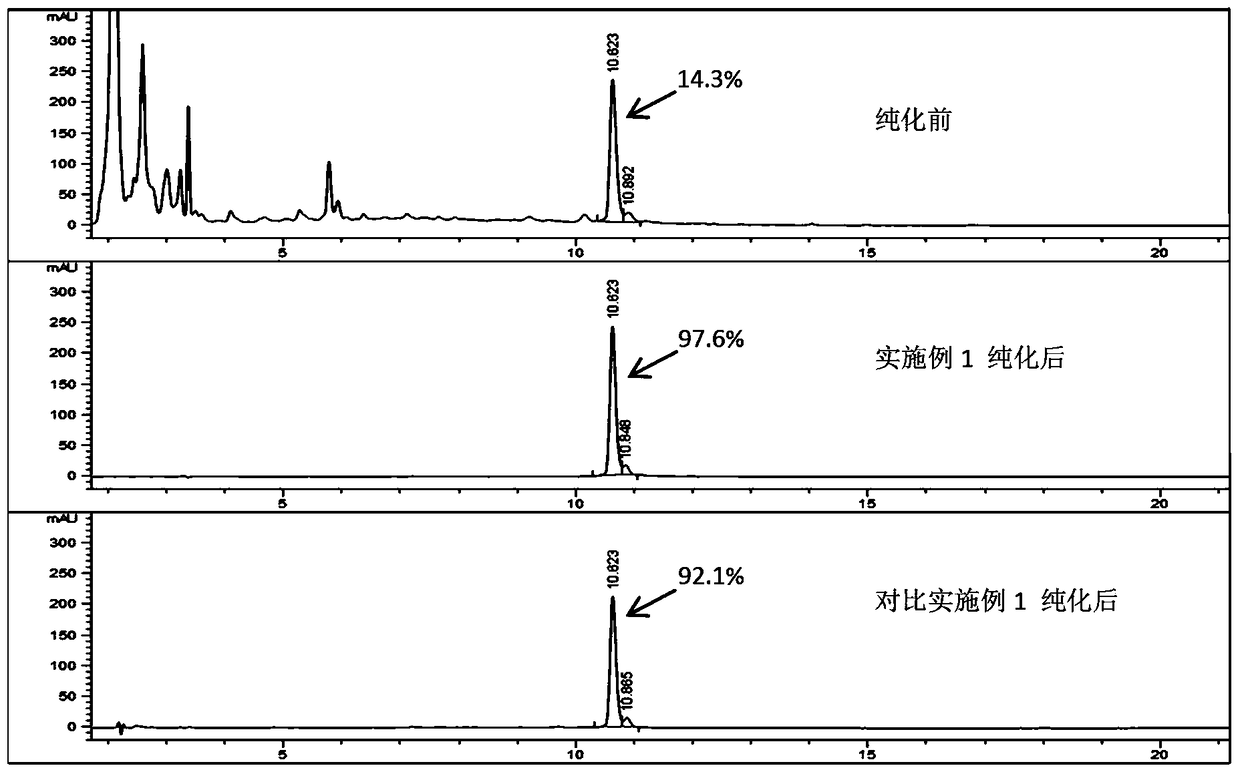
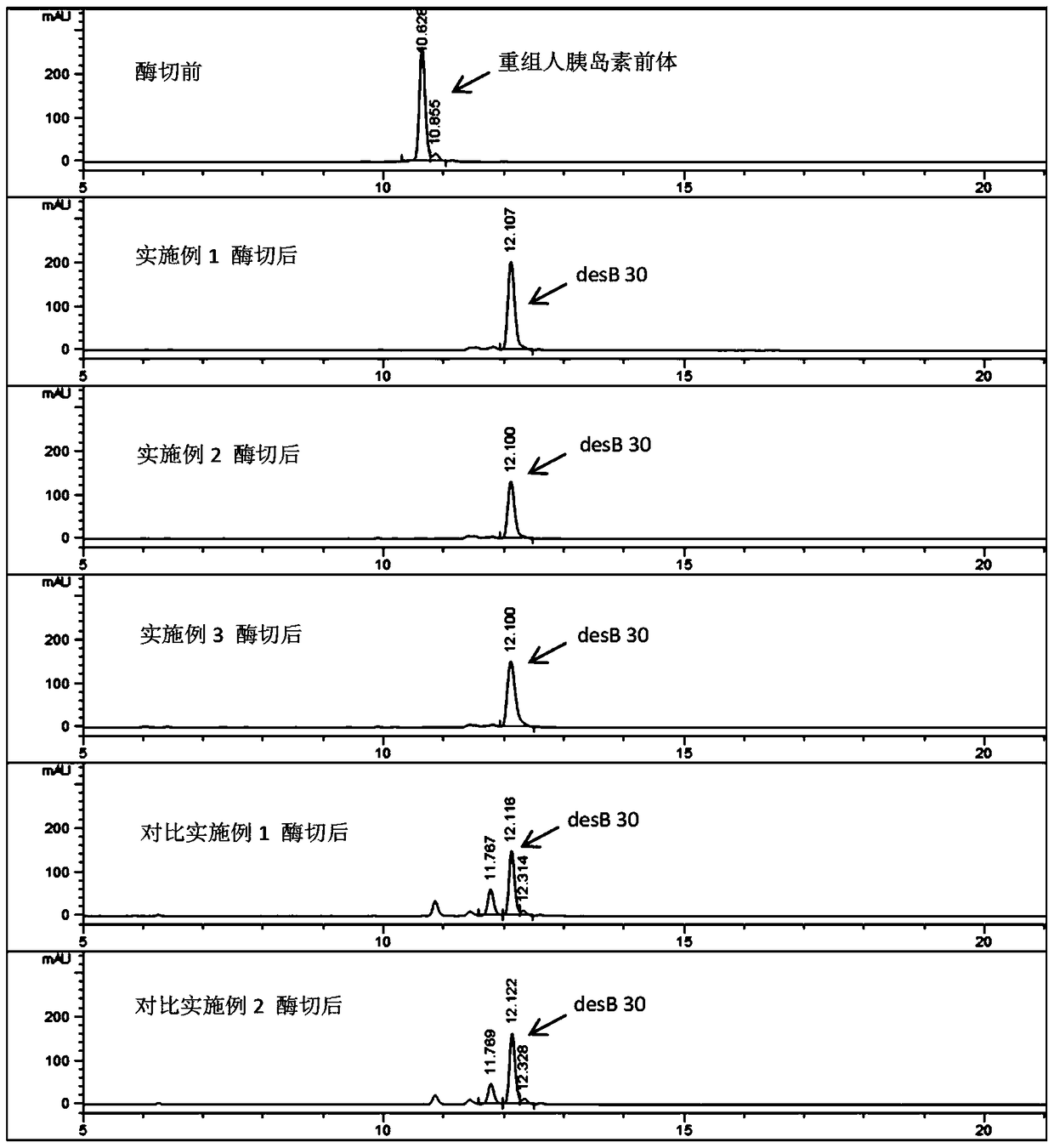
[0052] Example 2 Purification and enzymatic conversion method of recombinant human insulin precursor
[0053] The loading sample is the fermentation supernatant of Preliminary Example 1. The fermentation supernatant is diluted to 10 times the original volume with deionized water, and an appropriate amount of acetic acid is dissolved in the diluted sample to a final concentration of 20 mmol / L.
[0054] The purification method of ion chromatography adopts S Sepharose FF (XK 26 / 200, 50ml) on the chromatography workstation; the balance liquid is 20mmol / L acetic acid+0.01mol / L NaCl, and the washing liquid A is 20mmol / L acetic acid+0.1mol / L NaCl, washing liquid B is 5mmol / L hydrochloric acid, and eluent is 0.15mol / L boric acid buffer;
[0055] All solutions except the eluent (including loading sample, balance solution, washing solution A, and washing solution B) were adjusted to pH 3.5 with hydrochloric acid or sodium hydroxide, the pH of the eluent was adjusted to 8.5, and the col...
Embodiment 3
[0065] Example 3 Purification and enzymatic conversion method of recombinant human insulin precursor
[0066] The loading sample is the fermentation supernatant of Preliminary Example 1, and the fermentation supernatant is diluted to 10 times the original volume with deionized water. Measure an appropriate amount of acetic acid and add it to the diluted sample to a final concentration of 20mmol / L.
[0067] The purification method of ion chromatography adopts SP Sepharose FF (XK 26 / 200, 50ml) on the chromatography workstation; the balance liquid is 20mmol / L acetic acid+0.01mol / L NaCl, and the washing liquid A is 20mmol / L acetic acid+0.1mol / L NaCl, the washing solution B is 5mmol / L hydrochloric acid, and the eluent is 0.1mol / L tris (Tris);
[0068] All solutions except the eluent (including loading sample, balance solution, washing solution A, and washing solution B) were adjusted to pH 4.0 with hydrochloric acid or sodium hydroxide, and the pH of the eluent was adjusted to 8.0 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com