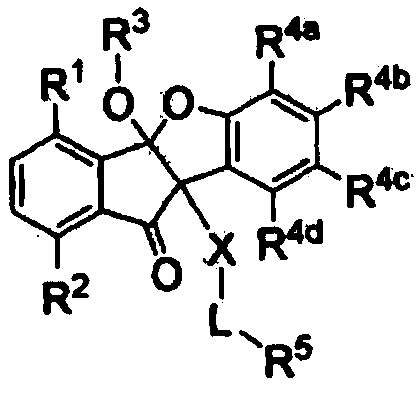
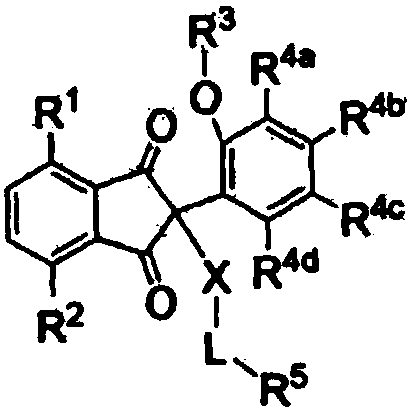
Antiviral compounds, pharmaceutically acceptable salts or optical isomers thereof, methods for producing them, and pharmaceutical compositions for preventing or treating viral diseases comprising them as active ingredients
A compound, pharmaceutical technology applied to antiviral compounds, pharmaceutically acceptable salts or optical isomers thereof, pharmaceutical compositions for preventing or treating viral diseases for preparing them and containing them as active ingredients In the field, it can solve the problems of side effects, unapproved antiviral drugs, and low treatment success rate, and achieve the effect of low cytotoxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0191] N-(4b-hydroxy-7-isopropyl-10-oxo-9b, 10-dihydro-4bH-benzo[d]indeno[1,2-b]furan-9b- Preparation of -2-(1H-indol-3-yl)-2-oxoacetamide
[0192] 2-(1H-indol-3-yl)-2-oxoacetic acid (352mg, 1.86mmol), EDCI (1-ethyl-3-(3-dimethylaminopropyl)carbodiimide) (355mg, 1.86mmol) and HOBt (hydroxybenzotriazole) (251mg, 1.86mmol) were dissolved in dichloromethane (MC) (10ml), then added 9b-amino-4b-hydroxy-7-isopropyl- 4bH-Benzo[d]indeno[1,2-b]furan-10(9bH)-one (500 mg, 1.69 mmol) and the mixture was stirred at room temperature for one day. The reaction mixture was extracted with dichloromethane to collect the organic layer, which was dissolved in MgSO 4 Dry under low temperature and concentrate under reduced pressure. The concentrated compound was purified by silica gel column chromatography (ethyl acetate:n-hexane=1:1) to obtain N-(4b-hydroxy-7-isopropyl-10-oxo-9b,10-dihydro-4bH-benzo [d] Indeno[1,2-b]furan-9b-yl)-2-(1H-indol-3-yl)-2-oxoacetamide (199 mg, 25%).
[0193] 1 H-NM...
Embodiment 2
[0195] N-(4b-hydroxy-7-isopropyl-10-oxo-9b, 10-dihydro-4bH-indeno[1,2-b]benzofuran-9b-yl)- Preparation of 2-oxo-2-(thiophen-2-yl)acetamide
[0196] After dissolving 2-oxo-2-(thiophen-2-yl)acetic acid (107mg, 1.07mmol) in DMF (3ml), the temperature was lowered to 0°C, and the solution was stirred. After 10 minutes, triethylamine (TEA) (213mg, 1.07mmol) and HATU (407mg, 1.07mmol) were added, stirred for 10 minutes before the temperature dropped to 0°C, and 9b-amino-4b-hydroxy-7-isopropyl yl-4bH-indeno[1,2-b]benzofuran-10(9bH)-one (300 mg, 1.02 mmol). Then, the temperature was raised to normal temperature, and the solution was stirred overnight. After washing with water, Na 2 SO 4 Remove moisture. After filtration and concentration, it was purified by column chromatography (EA:Hex=3:7) to obtain the title compound (52mg, 28%).
[0197] 1 H-NMR (300MHz, DMS0-d6) δ1.12 (d, 6H, J = 6.0Hz), 4.13 (q, 1H, J = 6.0Hz), 4.72 (s, 1H), 6.88 (d, 1H, J =6.0Hz),7.32(d,2H,J=6.0Hz),7.63...
Embodiment 3
[0199] N-(4b-hydroxyl-7-isopropyl-10-oxo-9b, 10-dihydro-4bH-indeno[1,2-b]benzofuran-9b-yl)- Preparation of 3-(2-nitrophenyl)-2-oxopropionamide
[0200] 2-Nitrophenylpyruvate (390mg, 1.86mmol) was placed in DMF:DCM (1:2, 15ml), and EDCI (487mg, 2.54mmol) was added at 0°C. Then, after adding 1-hydroxybenzotriazole (343 mg, 2.54 mmol), the mixture was stirred at room temperature for 15-30 minutes. Then, after adding 9b-amino-4b-hydroxyl-7-isopropyl-4bH-indeno[1,2-b]benzofuran-10(9bH)-one (500mg, 1.69mmol), TEA was added ( 0.709ml, 5.09mmol). After stirring at 60°C for 2 days, water (100ml) was added. The separated organic layer was collected and ethyl acetate (70ml x 3), washed with water (50ml) and brine (50ml), and the water was removed with sodium sulfate. After concentration, it was purified by silica gel column chromatography (25% ethyl acetate: hexane) to obtain 100 mg (24%) of the title compound.
[0201] 1 H-NMR (300MHz, CDC1 3 )δ1.18(dd, J=2.7Hz, J=6.9Hz, 6H, CH3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com