Controllable preparation method of multielement sulfide semiconductor nano-material
A nanomaterial, sulfide technology, applied in chemical instruments and methods, iron sulfide, tin compounds, etc., to achieve the effect of simple process and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] 1) Weigh 100 mg of ferrous chloride and 10 g of octadecylamine into a 50 ml four-neck flask, add a rotor and stir to dissolve.
[0039] 2) Weigh 100mg of sulfur powder and dissolve in 2g of octadecylamine, heat to 100°C, stir to dissolve.
[0040] 3) Heat the solution in 1) to 120°C. After injecting the solution in 2) into the solution in 1), continue heating to 220°C and keep at this temperature for 180min.
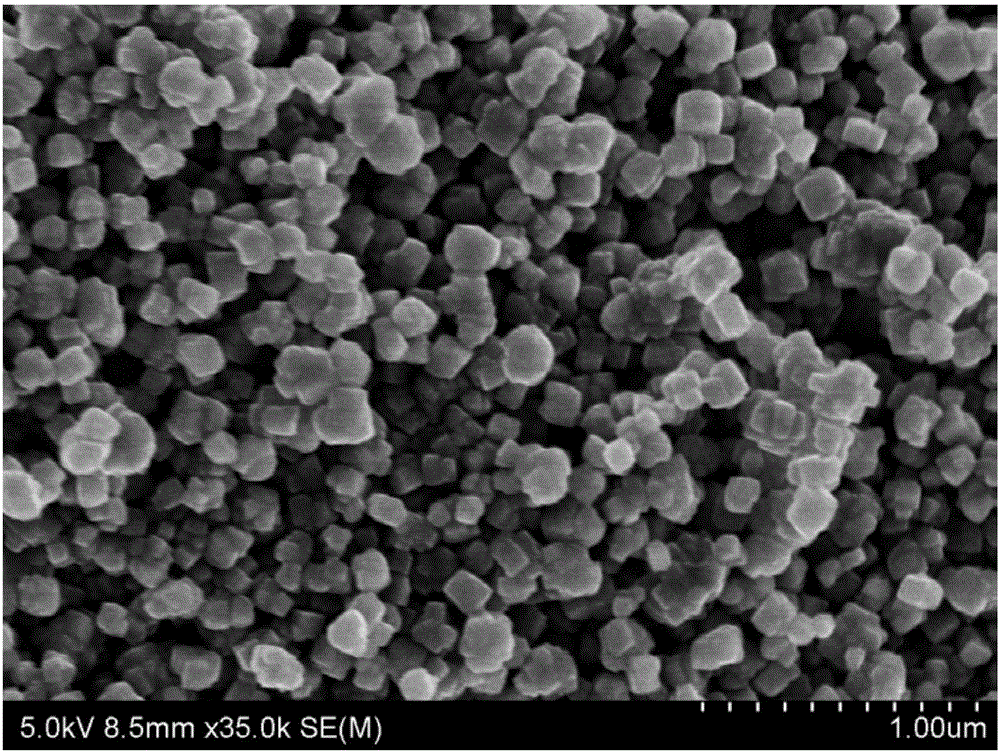
[0041] 4) After the system was cooled to room temperature, the resulting solution was washed with chloroform and centrifuged at 6000 rpm for 3 minutes. Repeat washing and centrifugation until the final centrifugation liquid is clear and transparent, and then put the obtained black solid in an oven at 80°C for 6 hours to obtain FeS 2 nanoparticles.
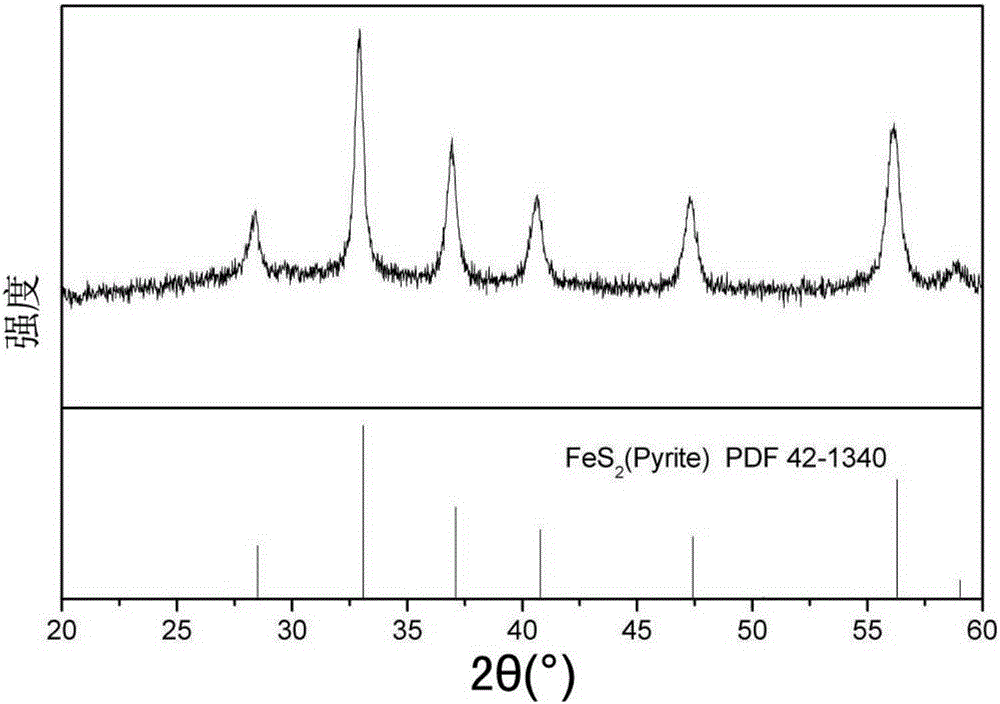
[0042] The phase composition identification of the material prepared in Example 1 was carried out by X-ray diffractometer. Such as figure 1, the 2θ angle positions of the peaks in the figure are 28.5°, 33.1°, 37.1...
Embodiment 2
[0045] 1) Weigh 278mg of ferric chloride and 15g of oleylamine into a 50ml four-neck flask, add a rotor and stir to dissolve.
[0046] 2) Weigh 192mg of sulfur powder and dissolve in 2g of oleylamine, heat to 90°C and stir to dissolve.
[0047] 3) Heat the solution in 1) to 220°C, inject the solution in 2) into the solution in 1), and keep at this temperature for 60 minutes after the temperature rises to 220°C.
[0048] 4) After the system was cooled to room temperature, the obtained solution was washed with n-hexane and centrifuged at 8000 rpm for 5 minutes. Repeat washing and centrifugation until the final centrifugation liquid is clear and transparent, and then put the obtained black solid in an oven at 80°C for 3 hours to obtain FeS 2 nanoparticles.
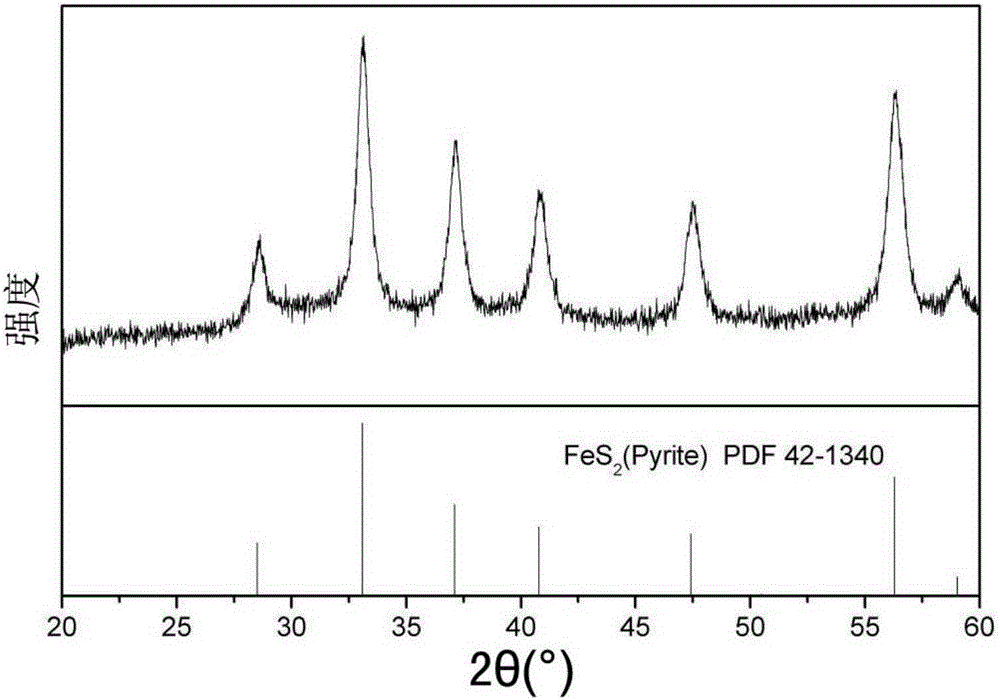
[0049] The phase composition identification of the material prepared in Example 2 was carried out by X-ray diffractometer. Such as image 3 , the 2θ angle positions of the peaks in the figure are 28.5°, 33.1°, 37.1°, 40.8...
Embodiment 3
[0052] 1) Weigh 278mg of ferric chloride and 10g of oleylamine into a 50ml four-neck flask, add a rotor and stir to dissolve.
[0053] 2) Weigh 256mg of sulfur powder and dissolve in 4g of octadecene, heat to 100°C and stir to dissolve.
[0054] 3) Heat the solution in 1) to 220°C, inject the solution in 2) into the solution in 1), and keep at this temperature for 30 minutes after the temperature rises to 220°C.
[0055] 4) After the system was cooled to room temperature, the obtained solution was washed with n-hexane and centrifuged at 6000 rpm for 3 minutes. Repeat washing and centrifugation until the final centrifugation liquid is clear and transparent, and then put the obtained black solid in an oven at 70°C for 4 hours to obtain FeS 2 nanoparticles.
[0056] The phase composition identification of the material prepared in Example 3 was carried out by X-ray diffractometer. Such as Figure 5 , the 2θ angle positions of the peaks in the figure are 28.5°, 33.1°, 37.1°, 40...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com