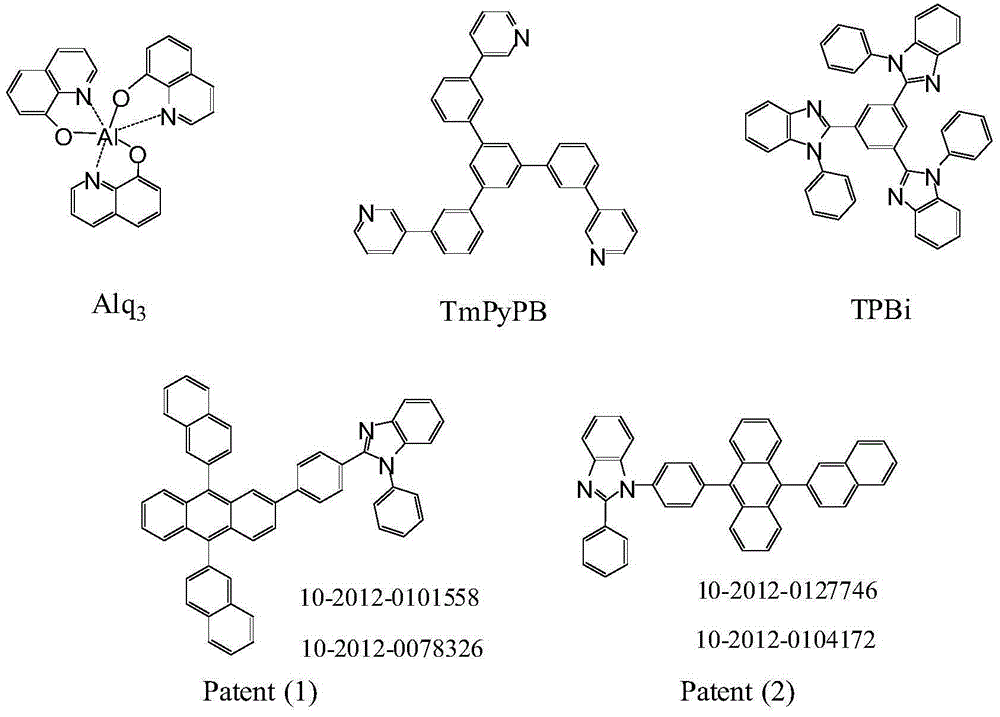
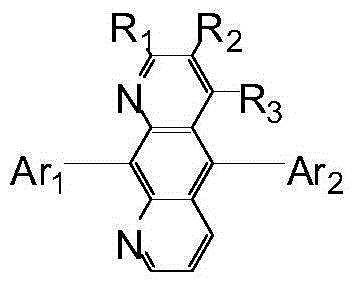
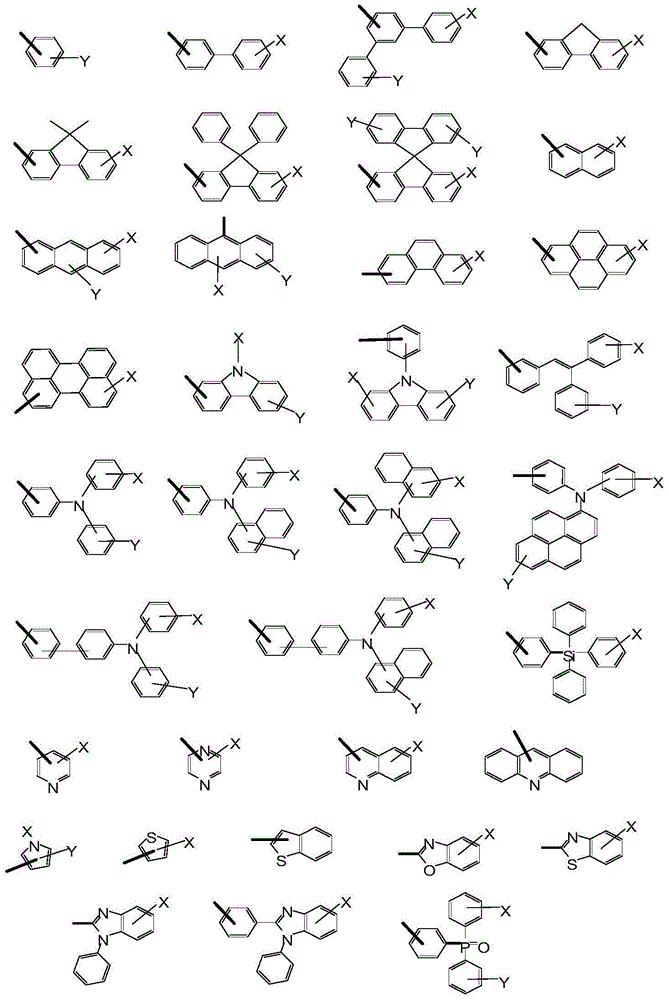
Novel aromatic amine compound and preparation and application thereof
A technology of aromatic amines and compounds, applied in the field of new aromatic amine compounds and their preparation and application, which can solve the problems of general material performance and achieve the effects of long life, high brightness and high efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0084] Example: Synthesis of Intermediate
[0085] Synthesis of the intermediate 2-chloro-6-phenylpyridine (1-1)
[0086]
[0087] Synthesis method: 2-bromo-6-chloropyridine (21.2g, 0.11mol), phenylboronic acid (12.2g, 0.10mol), 0.5g tetrakistriphenylphosphorus palladium, add to a 1000ML reaction flask, add 400ML toluene, carbonic acid The sodium aqueous solution (2N, 150 mL) was protected by nitrogen and reacted in an oil bath at 80°C for 24 hours. Post-treatment process: cool down, stand still for 30 minutes to separate liquids, save the organic layer, spin dry toluene, add methylene chloride to the solid to dissolve, pass through the column for separation, PE:DCM=1:1, get A-1(9.6g, y=51%)
[0088] Synthesis of intermediates 1-2 and 1-3
[0089] According to the above-mentioned synthesis method of Intermediate 1-1, the following compounds are obtained:
[0090]
[0091] Synthesis of intermediate 6-chloro-N,N-diphenylpyridine-2-amine (1-4):
[0092]
[0093] Synthesis method: add dip...
Embodiment 5
[0125] Example 5 Synthesis of 10-diphenylpyrido[3,2-g]quinoline(6-1)
[0126]
[0127] Add the prepared 5,10-dichloro-pyrido[g]quinoline 5-1 (14.8g, 0.6mmol), 4-boronic acid pyridine (18g, 0.146mmol), and 4g of tetratriphenylphosphine palladium into the reaction flask Add toluene, ethanol and water 2:1:1 (volume ratio) mixed solution totaling 600mL, nitrogen protection, stirring and heating to 110°C for 24 hours. Post-treatment process: cooling system, liquid separation, spin-dry toluene. Add dichloromethane to dissolve the solid, pass the column, and wash with petroleum ether: ethyl acetate=2:1 (volume ratio) to obtain a solid (6-1) (13g, y=65%).
Embodiment 6-2~6-17
[0128] Synthesis of Examples 6-2~6-17
[0129] According to the synthesis method of Example 6-1 above, the compounds in the following table were obtained:
[0130]
[0131]
[0132]
[0133]
[0134] Example N5, N5, N10, N10-Tetraphenylpyrido[3,2-g]quinoline-5,10-diamine (6-23) synthesis:
[0135]
[0136] According to the synthesis method of Intermediate 1-4, 5,10-dichloropyrido[3,2-g]quinoline (6.0g, 24mmol) and diphenylamine (4.4, 24mmol) were used as raw materials for the reaction to obtain N5, N5, N10, N10-tetraphenylpyrido[3,2-g]quinoline-5,10-diamine (6.17g, y=50%).
[0137] Example 5 Synthesis of 10-bis(1-naphthyloxy)pyrido[3,2-g]quinoline (6-24):
[0138]
[0139] Dissolve 1-hydroxynaphthalene (14g, 0.1mol) in 100mL of anhydrous tetrahydrofuran, stir, accurately weigh NaH (0.96g, 0.4mol) and add them to the reaction flask in batches, not too fast to prevent too many bubbles. After the addition, the solution turned yellow, and then 5,10-dichloropyrido[3,2-g]quinoline (27.50g, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com