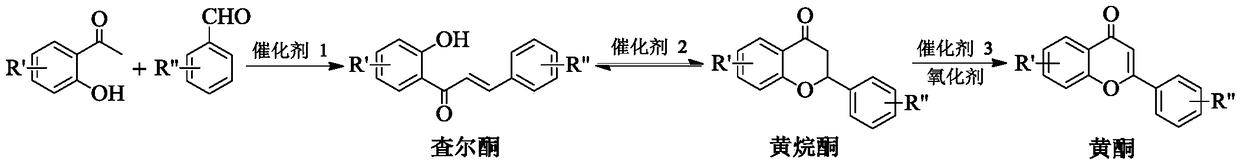
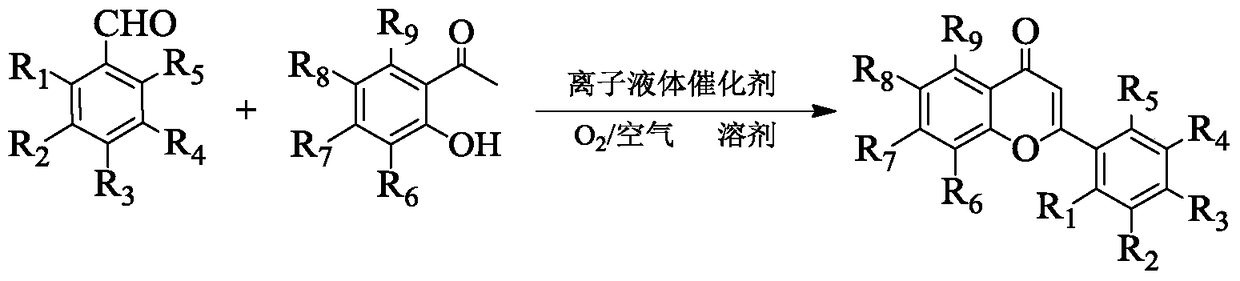
A method for one-step synthesis of flavonoids catalyzed by 1,3-dialkylimidazolium oxometalates
A technology for flavonoids and dialkylimidazoles, which is applied in the field of synthesizing flavonoids, can solve the problems of low yield of flavonoids, complicated operation and the like, and achieves the effects of improving synthesis efficiency, concise operation steps and high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1: the synthesis of flavonoids
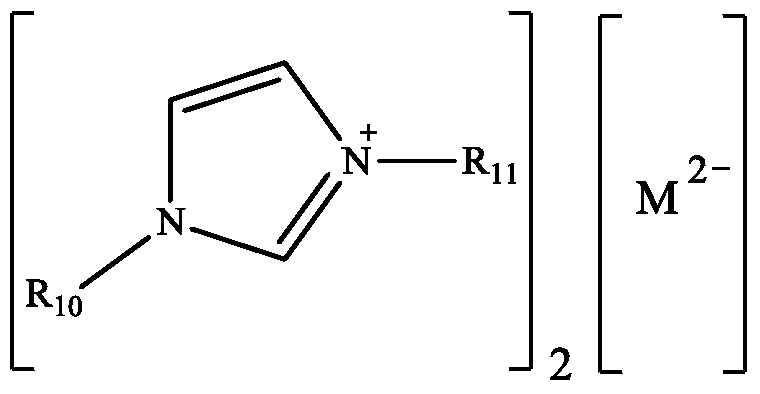
[0026] Ionic liquid catalyst 1-butyl-3-methylimidazolium molybdate (abbreviated as [Bmim] 2 [MoO 4 ]), its structural formula is:
[0027]
[0028] In a 10mL reactor, add 0.2122g (2mmol) benzaldehyde, 0.2723g (2mmol) 2-hydroxyacetophenone, 0.8174g (8mmol) solvent n-hexanol and 0.2630g (0.6mmol) ionic liquid catalyst [Bmim] successively 2 [MoO 4 ], stir and mix evenly, heat up to the reaction temperature of 70°C, stir and react in an open environment for 3h, cool to room temperature after the reaction, add water for extraction, stand for phase separation, and remove the solvent n-hexanol from the upper phase by vacuum distillation , and then separated by column chromatography and recrystallization to obtain the target product flavone, the yield is 98%, and its structural formula is determined by proton nuclear magnetic resonance spectrum, carbon spectrum and high resolution mass spectrometry:
[0029]
[0030] The low...
Embodiment 2
[0031] Embodiment 2: the synthesis of 4'-methylflavone
[0032] Ionic liquid catalyst 1-ethyl-3-methylimidazolium vanadate (abbreviated as [Emim] 2 [VO 4 ]), its structural formula is:
[0033]
[0034] In a 10mL reactor, add 0.2403g (2mmol) 4-methylbenzaldehyde, 0.2723g (2mmol) 2-hydroxyacetophenone and 0.2024g (0.6mmol) ionic liquid catalyst [Emim] 2 [VO 4 ], and 0.4085g (4mmol) tetrahydrofurfuryl alcohol solvent, after stirring and mixing uniformly, heat up to the reaction temperature of 50°C, stir and react for 3h under an oxygen atmosphere of 0.1MPa, cool to room temperature after the reaction, and remove the solvent by distillation under reduced pressure After tetrahydrofurfuryl alcohol, add ethyl acetate and water to extract, let stand to separate the phases, and then separate the upper phase through column chromatography and recrystallization to obtain the target product 4'-methylflavone with a yield of 95%. , carbon spectrum and high-resolution mass spectrometry ...
Embodiment 3
[0036] Embodiment 3: the synthesis of 2'-chloroflavone
[0037] Ionic liquid catalyst 1-propyl-3-methylimidazolium vanadate (abbreviated as [Pmim] 2 [VO 4 ]), its structural formula is:
[0038]
[0039] In a 10mL reactor, add 0.2811g (2mmol) 2-chlorobenzaldehyde, 0.2723g (2mmol) 2-hydroxyacetophenone and 0.1461g (0.4mmol) ionic liquid catalyst [Pmim] 2 [VO 4 ], and 0.3724g (6mmol) ethylene glycol solvent, after stirring and mixing evenly, heat up to the reaction temperature of 60°C, stir and react for 3h under an oxygen atmosphere of 0.1MPa, cool to room temperature after the reaction, and remove the solvent by distillation under reduced pressure After ethylene glycol, add ethyl acetate and water for extraction, let stand to separate the phases, and then separate the upper phase through column chromatography and recrystallization to obtain the target product 2'-chloroflavone with a yield of 94%. Carbon spectrum and high-resolution mass spectrometry determine that its s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com