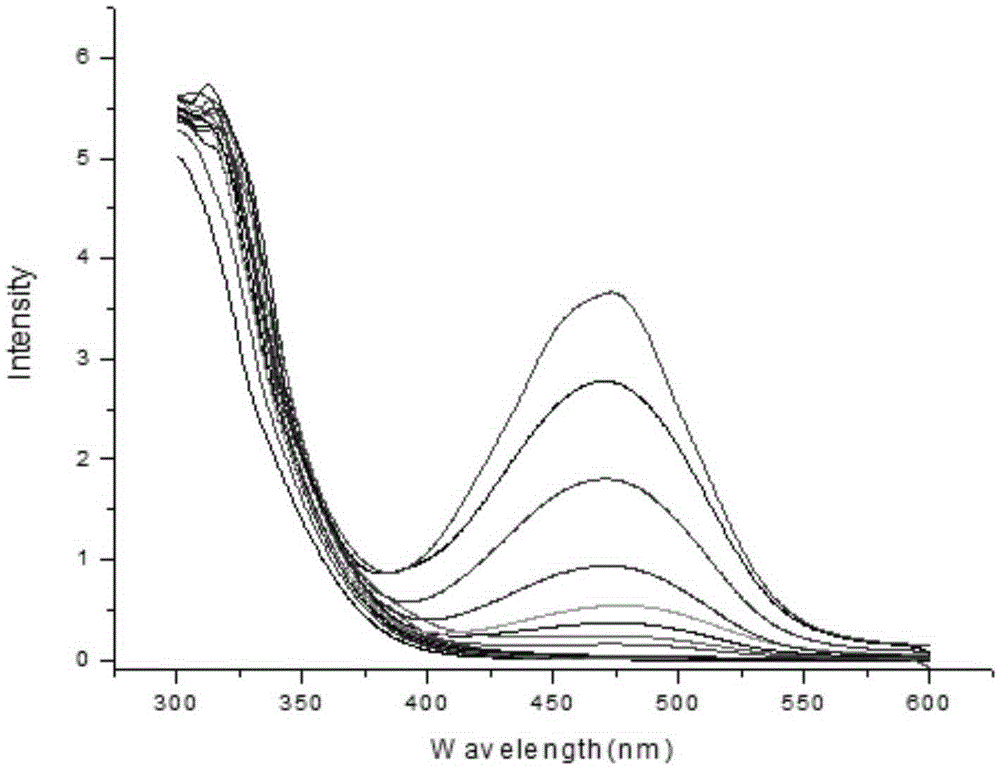
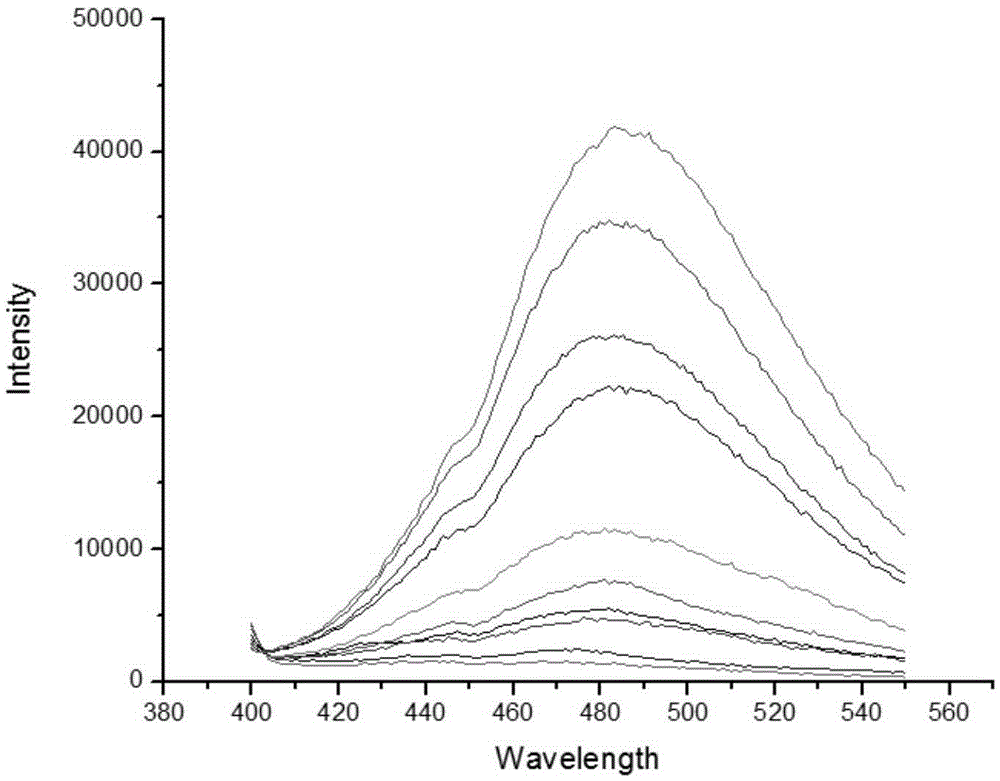
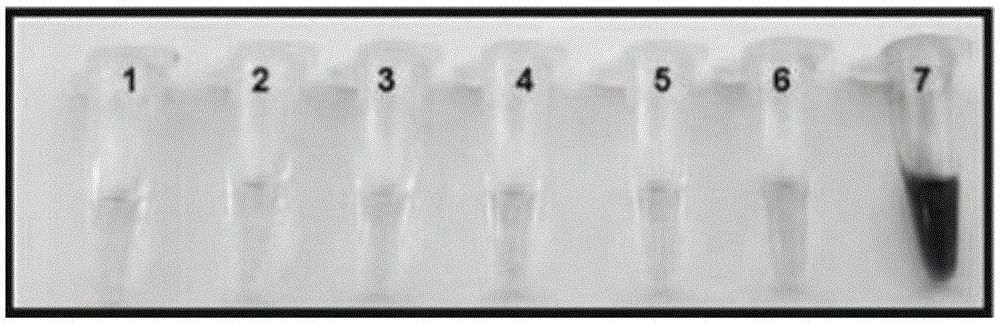
Fluorescence probe rapidly responding to hydrogen sulfide and preparation method and application thereof
A fluorescent probe and fast-response technology, applied in the field of fluorescent probe and its preparation, achieves low self-absorption, improved imaging accuracy, and good dyeing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] In a 25ml two-necked flask, add hydroxytetraphenylethylene (0.364g, 1mmol), 2,4-dinitrobromobenzene (0.738g, 3mmol), activated potassium carbonate (0.414g, 3mmol), and vacuum— - Nitrogen was passed three times, water-removed DMF was added, the temperature was raised to 90° C., and the reaction was carried out for 4 hours. The solution turned yellow. After the reaction, the reaction liquid was poured into the ice-water mixture, the product was precipitated rapidly, and the mixed liquid was milky, extracted with a large amount of ethyl acetate, and the ethyl acetate phase was a yellow liquid. Add anhydrous magnesium sulfate to dry. Magnesium sulfate was filtered off, spin-dried, and passed through the column. The eluent is 5 to 1 petroleum ether to dichloromethane, R f The value is about 0.25. After being passed through the column and spin-dried, a light yellow solid (0.341 g, 0.49 mmol) was obtained with a yield of 49%.
Embodiment 2
[0044] In a 25ml two-necked flask, add hydroxytetraphenylethylene (1mmol), 2,4-dinitrobromobenzene (2mmol), activated potassium carbonate (0.414g, 3mmol), vacuumize---pass nitrogen three times, add DMF was removed, the temperature was raised to 85°C, and the reaction was carried out for 5 hours. The solution turned yellow. After the reaction, the reaction liquid was poured into the ice-water mixture, the product was precipitated rapidly, and the mixed liquid was milky, extracted with a large amount of ethyl acetate, and the ethyl acetate phase was a yellow liquid. Add anhydrous magnesium sulfate to dry. Magnesium sulfate was filtered off, spin-dried, and passed through the column. The eluent is 5 to 1 petroleum ether to dichloromethane, R f The value is about 0.25. After passing through the column, it was spin-dried to obtain a light yellow solid with a yield of 45%.
Embodiment 3
[0046] In a 25ml two-neck flask, add hydroxytetraphenylethylene (1mmol), 2,4-dinitrobromobenzene (4mmol), activated potassium carbonate (3mmol), vacuumize --- pass nitrogen three times, add dehydrated DMF , heated up to 95°C, and reacted for 4 hours. The solution turned yellow. After the reaction, the reaction liquid was poured into the ice-water mixture, the product was precipitated rapidly, and the mixed liquid was milky, extracted with a large amount of ethyl acetate, and the ethyl acetate phase was a yellow liquid. Add anhydrous magnesium sulfate to dry. Magnesium sulfate was filtered off, spin-dried, and passed through the column. The eluent is 5 to 1 petroleum ether to dichloromethane, R f The value is about 0.25. After passing through the column, it was spin-dried to obtain a light yellow solid with a yield of 42%.
[0047] Characterization of melting point, infrared, NMR and mass spectrometry:
[0048] Probe melting point (m.p.):224-225℃
[0049] 1 HNMR (400MHz...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com