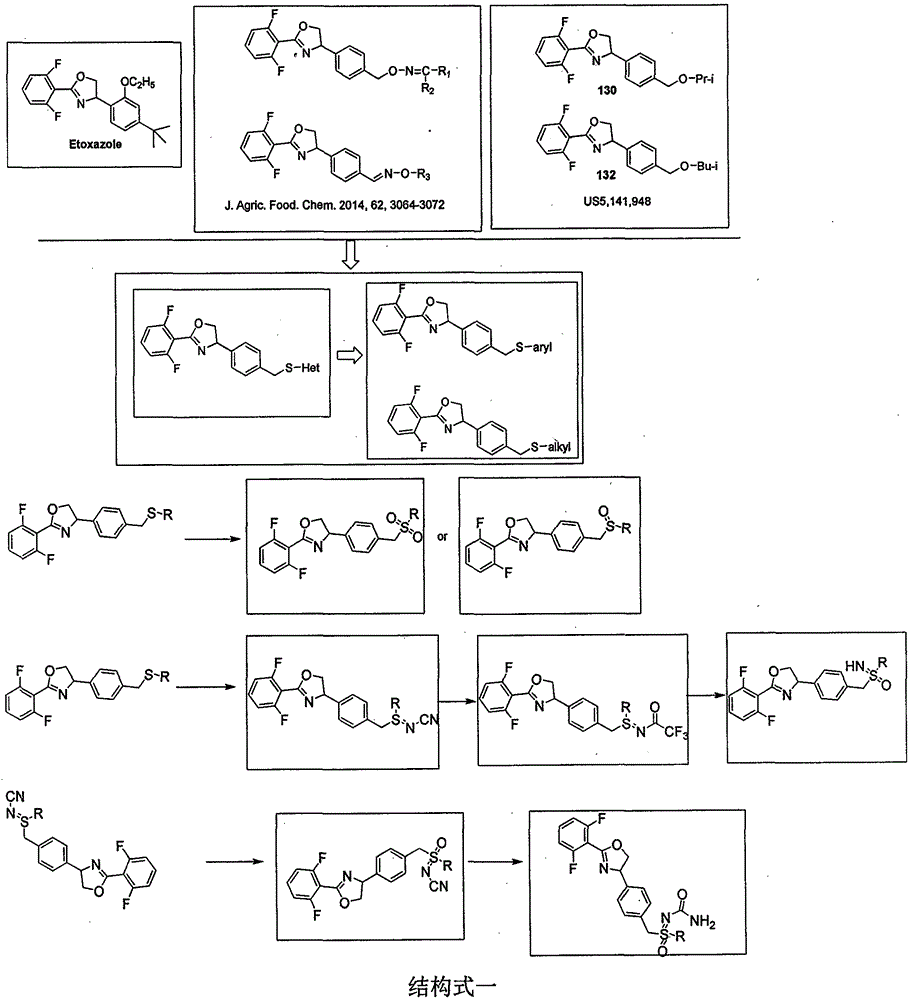
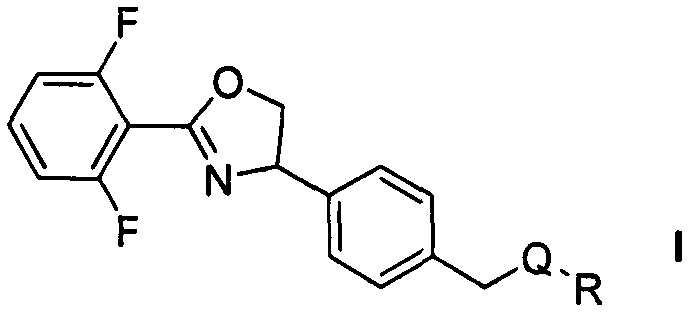
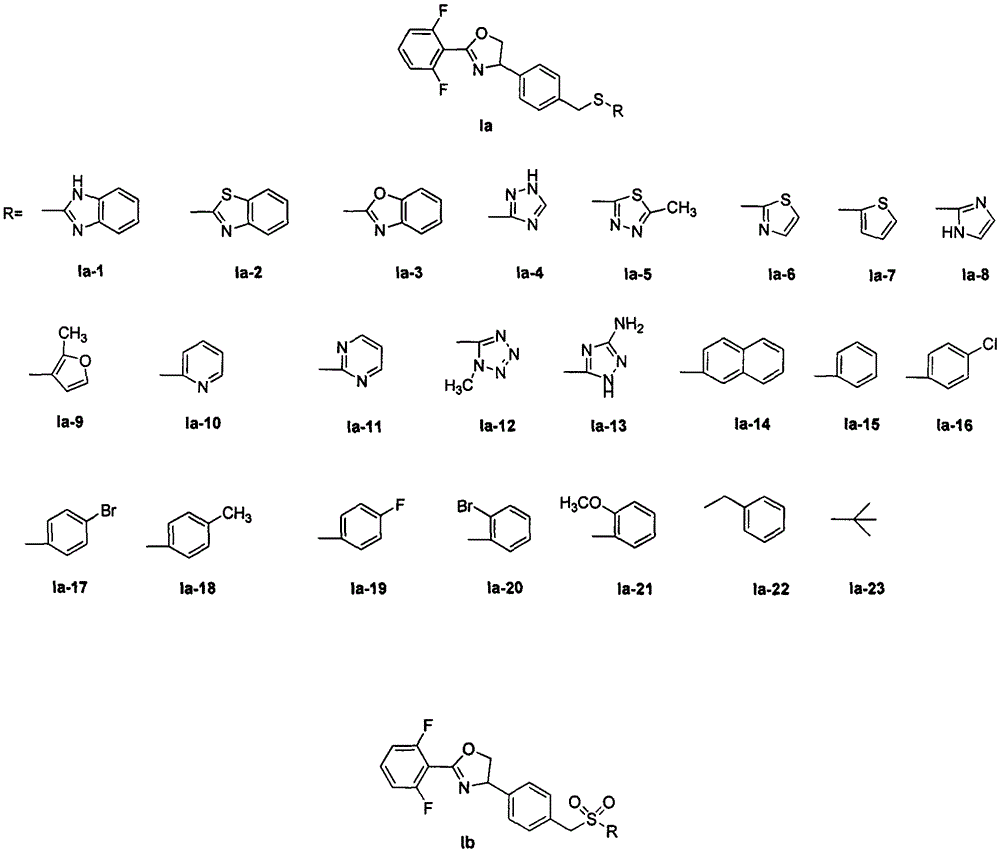
Oxazoline compound with 4-phenyl para-position containing thioether and derivative structure thereof, preparation and application as pest and mite killing agent
A technology of oxazolines and compounds, which is applied in the field of oxazolines compounds, can solve problems such as human body and environmental damage, and achieve excellent killing and activity effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1: the synthesis of compound Ia-1:
[0024]5.0g (16.1mmol) of compound f, 40mL of dichloromethane, 40mL of water, 1.9g (16.1mmol) of potassium bromide and 1.33g (8.05mmol) of potassium bromate were successively added into a 250mL single-necked bottle. Control the temperature in an ice-water bath at 0°C, add 20 mL of a solution containing 6.1 mL of concentrated hydrochloric acid dropwise, react for 25 hours, monitor the completion of the reaction by TLC, separate the liquids, and wash the organic phase with sodium carbonate solution and sodium bicarbonate solution until it is nearly colorless, and then use The aqueous phase was extracted with dichloromethane, and the combined organic phases were washed with sodium chloride solution, anhydrous NaSO 4 Dry, filter with suction, and spin dry to obtain a white solid, which is recrystallized from toluene and petroleum ether to obtain the product g, a total of 3.5 g, with a yield of 55.9%. Melting point: 127-128°C. ...
Embodiment 2
[0026] Embodiment 2: the synthesis of compound Ia-2~Ia-23:
[0027] Compounds Ia-2 to Ia-23 were synthesized by the method shown in Example 1.
[0028] Compound Ia-2
[0029] Pale yellow solid, yield 57%. Melting point: 92-94°C. 1 HNMR (400MHz, CDCl 3 )δ7.91(d, J=8.0Hz, 1H), 7.76(d, J=8.0Hz, 1H), 7.48(d, J=8.4Hz, 2H), 7.46-7.41(m, 3H), 7.32( d, J=8.4Hz, 2H), 7.00(t, J=8.0Hz, 2H), 5.46(dd, J=10.4, 8.4Hz, 1H), 4.81(dd, J=10.4, 8.4Hz, 1H), 4.61(s, 2H), 4.29(t, J=8.4Hz, 1H); 13 CNMR (100MHz, CDCl 3 )δ166.3, 161.2 (dd, J=254.8, 6.0Hz), 157.7, 153.2, 141.4, 135.8, 135.4, 132.5 (t, J=10.4Hz), 129.6, 127.1, 126.1, 124.3, 121.6, 121.0, 112.0 (dd, J=22.8, 2.6Hz), 107.2 (t, J=17.4Hz), 74.7, 70.0, 37.4; HRMS(ESI)calcdforC 23 h 17 f 2 N 2 OS 2 [M+H] + 439.0745, found 439.0751.
[0030] Compound Ia-3
[0031] Yellow solid, yield 71%. Melting point: 110-112°C. 1 HNMR (400MHz, CDCl 3 )δ7.62(d, J=7.6Hz, 1H), 7.48(d, J=8.0Hz, 2H), 7.46-7.40(m, 2H), 7.32(d, J=8.0Hz, 2H), 7.24- ...
Embodiment 3
[0072] Embodiment 3: the synthesis of compound Ib-1:
[0073] Add 2-(2,6-difluorophenyl)-4-(4-((naphthalene-2-ylsulfur)methyl)phenyl)-4,5-dihydrooxazole Ia- 14 (0.5g, 1.16mmol), 20mL of dichloromethane, 1.25mL of trifluoroacetic acid was added under stirring at room temperature, 0.5mL of 30% hydrogen peroxide was added in batches, the reaction was carried out at room temperature, and the reaction progress was tracked by TLC. Add 1.5 g of sodium bisulfite to decompose excess hydrogen peroxide, and stir at room temperature for 1 h. Add 20 mL of water, separate the layers, extract the aqueous phase with 2×20 mL of dichloromethane, combine the organic phases, wash with 15 mL of water and 15 mL of brine in turn, and dry over anhydrous sodium sulfate. Filtration, precipitation, crude silica gel column chromatography [eluent is petroleum ether (60-90°C) and ethyl acetate = 4:1], to obtain 0.49g of white solid, yield 90%, melting point: 157-159°C . 1 HNMR (400MHz, CDCl 3 )δ8.23 (s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com