Composition with effects of protecting joints and increasing bone mineral density and preparation method of composition
A technology to increase bone density and protect joints, applied to medical preparations containing active ingredients, drug combinations, applications, etc., can solve problems such as increased bone fragility, prone to bone pain, fractures, and unable to fundamentally solve bone health
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
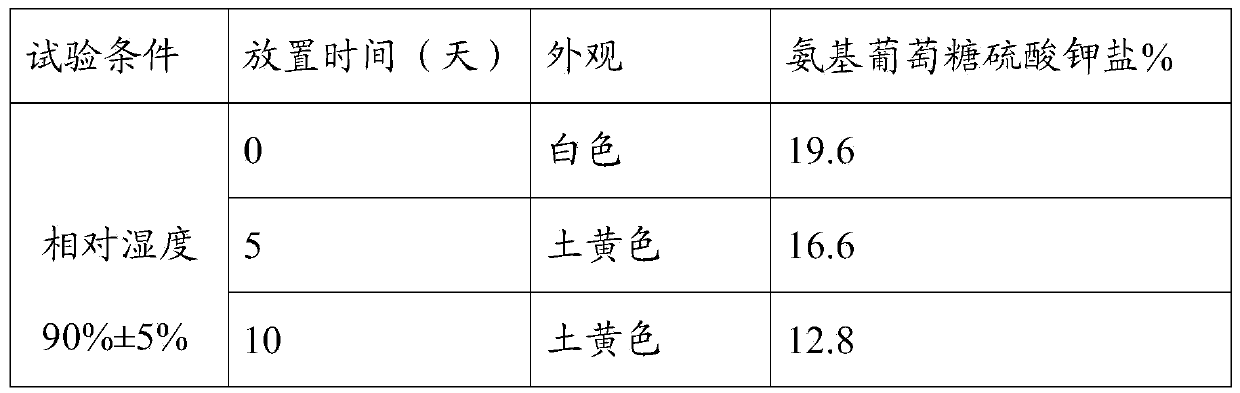
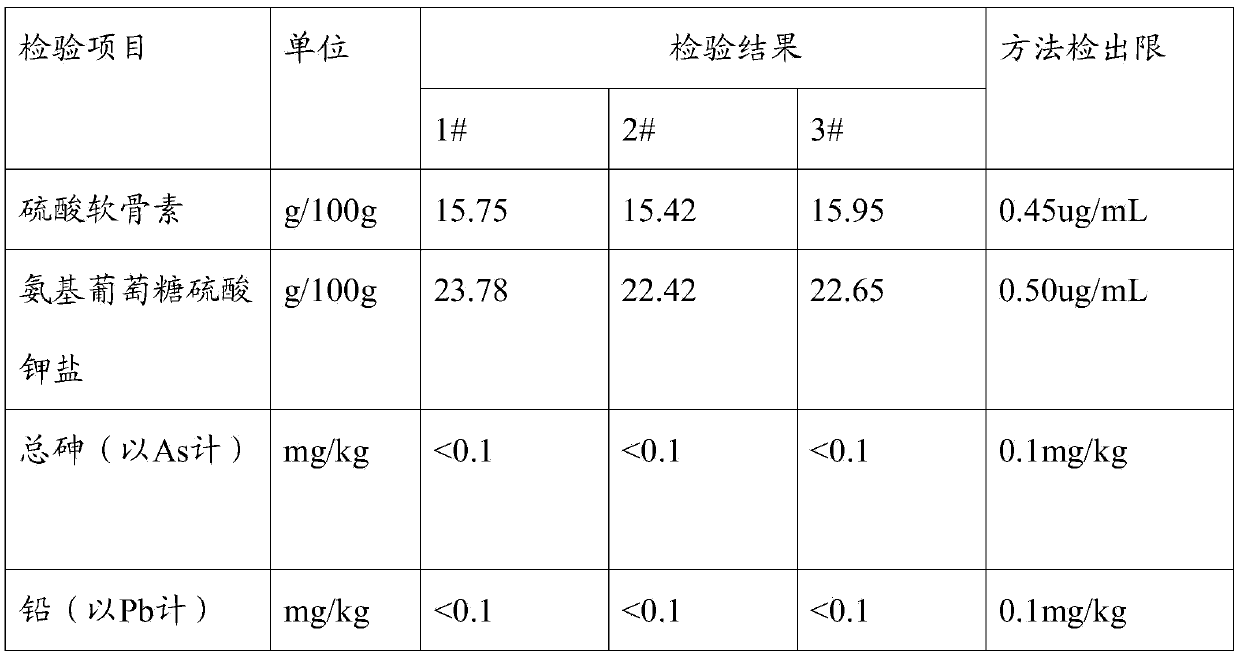
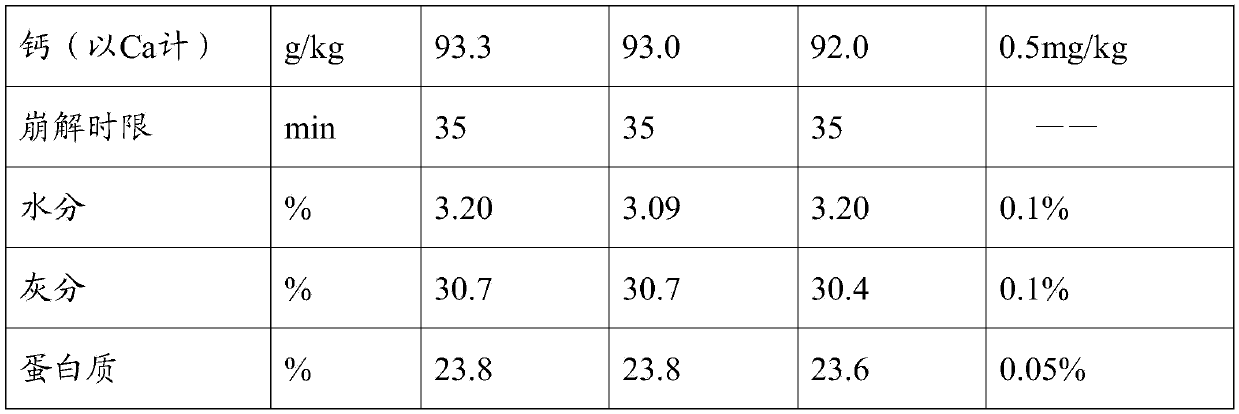
Image
Examples
Embodiment 1
[0071] Embodiment 1: preparation of composition tablet of the present invention
[0072] 1. Raw material composition:
[0073] Calcium citrate malate 330g, glucosamine sulfate potassium salt 220g, chondroitin sulfate 160g, bone collagen peptide powder 100g, casein phosphopeptide 30g.
[0074] 2. The auxiliary materials are composed of:
[0075] Lactose 50g, copovidone 50g, microcrystalline cellulose 40g, silicon dioxide 5g, magnesium stearate 5g.
[0076] 3. Preparation method:
[0077](1) Measure the moisture of each raw and auxiliary material, and control the moisture to ≤5.0%;
[0078] (2) Pass calcium citrate malate, collagen peptide powder, casein phosphopeptide, lactose, copovidone, and microcrystalline cellulose through a 60-mesh sieve; chondroitin sulfate and magnesium stearate pass through an 80-mesh sieve, press The formula weighs each raw and auxiliary material;
[0079] (3) Mix glucosamine sulfate potassium salt and silicon dioxide for 5 minutes, pass through...
Embodiment 2
[0082] Embodiment 2: preparation of composition tablet of the present invention
[0083] 1. Raw material composition:
[0084] Calcium citrate malate 400g, glucosamine sulfate potassium salt 150g, chondroitin sulfate 200g, bone collagen peptide powder 50g, casein phosphopeptide 40g.
[0085] 2. Composition of auxiliary materials:
[0086] Lactose 70g, copovidone 50g, microcrystalline cellulose 30g, silicon dioxide 5g, magnesium stearate 5g.
[0087] 3. Preparation method:
[0088] (1) Measure the moisture content of each raw and auxiliary material, and control the moisture content to ≤5.0%;
[0089] (2) Pass each raw and auxiliary material through a 60-mesh sieve, and weigh each raw and auxiliary material according to the formula;
[0090] (3) Mix glucosamine sulfate potassium salt and silicon dioxide for 30 minutes, and then mix with the above-mentioned other materials that have been sieved with an automatic lifting hopper mixer for 45 minutes, then put the materials in...
Embodiment 3
[0092] Embodiment 3: preparation of composition tablet of the present invention
[0093] 1. Raw material composition:
[0094] Calcium citrate malate 250g, glucosamine sulfate potassium salt 300g, chondroitin sulfate 100g, bone collagen peptide powder 150g, casein phosphopeptide 40g.
[0095] 2. Composition of auxiliary materials:
[0096] Lactose 30g, copovidone 50g, microcrystalline cellulose 55g, silicon dioxide 5g, magnesium stearate 10g.
[0097] 3. Preparation method:
[0098] (1) Measure the moisture content of each raw and auxiliary material, and control the moisture content to ≤5.0%;
[0099] (2) Pass each raw and auxiliary material through an 80-mesh sieve, and weigh each raw and auxiliary material according to the formula;
[0100] (3) Mix glucosamine sulfate potassium salt and silicon dioxide for 10 minutes, then mix with the above-mentioned other materials that have been sieved with an automatic lifting hopper mixer for 75 minutes, then put the materials int...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com