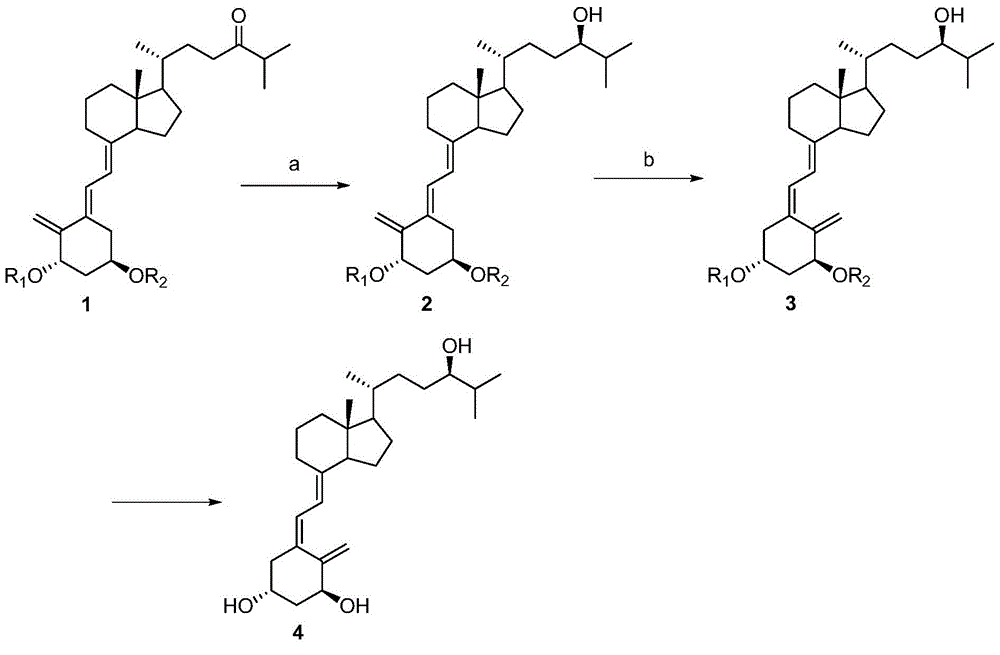
Method for enantioselective synthesis of tacalcitol
A technology of enantioselectivity and tacalcitol, which is applied in the field of preparation of enantioselective synthesis of tacalcitol, can solve the problems of costing a lot of manpower and material resources and equipment costs, and achieve the requirements of loose reaction conditions and high production efficiency. High efficiency and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Embodiment 1: the synthesis of compound 2
[0043] Under nitrogen protection, 2M tetrahydrofuran solution (0.2mL) of borane tetrahydrofuran complex was added dropwise to 1M (R)-2-methyl-CBS-oxazolidine solution (0.2mL) in toluene, at room temperature The reaction was stirred for 2 hours, cooled to -20°C, and a toluene solution of compound 1 (128mg, 0.2mmol) was slowly added, then stirred at -20°C for 0.5 hours, TLC showed that the reaction was complete, and ammonium chloride was added to the reaction system. aqueous solution, moved to room temperature and stirred for 0.5 hours, then poured the reaction system into an aqueous solution of ammonium chloride (30mL), extracted with ethyl acetate (3×20mL), washed the organic layer with water (2×20mL), and washed with saturated brine (2× 20 mL), dried over anhydrous sodium sulfate, filtered, and the residue was separated by Pre-HPLC to obtain enol 2 as a colorless solid (91 mg, 71%) after distilling off the solvent. 1 HNMR (5...
Embodiment 2
[0044] Embodiment 2: the synthesis of compound 3
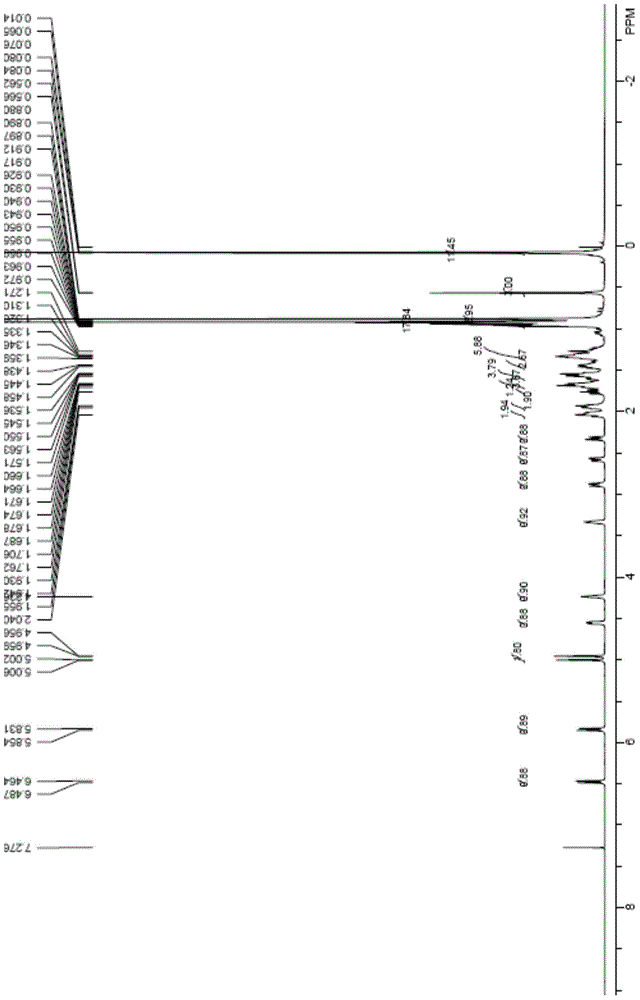
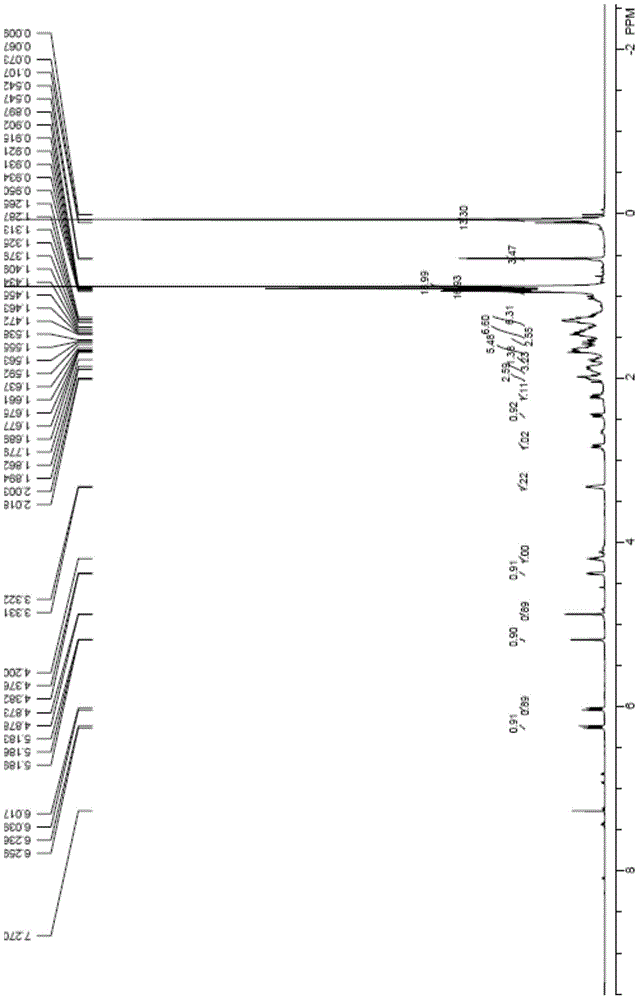
[0045] Compound 2 (1.29g, 2mmol) and 9-acetylanthracene (222mg, 1mmol) were dissolved in toluene (80mL), stirred and reacted under 365nm ultraviolet light irradiation at 20°C for 1.5 hours, after the completion of the reaction as detected by TLC, the reaction solution was concentrated in vacuo , and the residue was subjected to column chromatography (SiO 2 , V(PE):V(EA)=20:1) Compound 3 (0.92g, 71%) was isolated as a white solid. 1 HNMR (500MHz, CDCl 3 )δ6.24(d, J=11.2Hz, 1H), 6.02(d, J=11.3Hz, 1H), 5.18(s, 1H), 4.87(s, 1H), 4.37(m, 1H), 4.19( m,1H),3.32(m,1H),0.92(m,9H),0.88(s,18H),0.54(s,3H),0.07(s,12H).
Embodiment 3
[0046] Embodiment 3: the synthesis of compound 4
[0047] Compound 3 (645mg, 1mmol) was dissolved in tetrahydrofuran (30mL), and 1MTBAF solution in tetrahydrofuran (5mL, 5mmol) was added under the protection of argon, and the reaction was stirred at 60°C for 3 hours. After the reaction was detected by TLC, saturated Aqueous sodium bicarbonate solution (100mL), extraction with ethyl acetate (3×40mL), organic layer washed with water (2×30mL), washed with saturated brine (2×30mL), dried over anhydrous magnesium sulfate, filtered, spin-dried, and the residue Column chromatography (SiO 2 , V(PE):V(EA)=1:1) Compound 4 (tacalcitol) was obtained as a white solid (279 mg, 67%) after separation. 1 HNMR (500MHz, CDCl 3 )δ6.37(d, J=11.2Hz, 1H), 6.02(d, J=11.1Hz, 1H), 5.32(s, 1H), 4.99(s, 1H), 4.42(m, 1H), 4.22( m,1H),3.32(m,1H),0.92(m,9H),0.55(s,3H). 13 CNMR (125MHz, CDCl 3 )δ147.7, 143.1, 133.0, 124.9, 117.1, 111.8, 77.4, 70.7, 66.8, 56.3, 45.9, 45.2, 42.8, 40.5, 36.0, 33.6, 33.2, 3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com