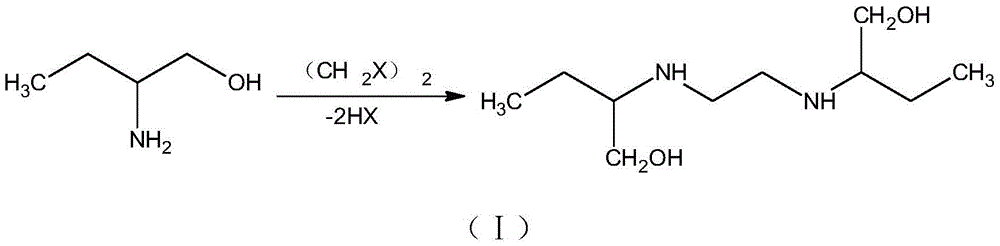
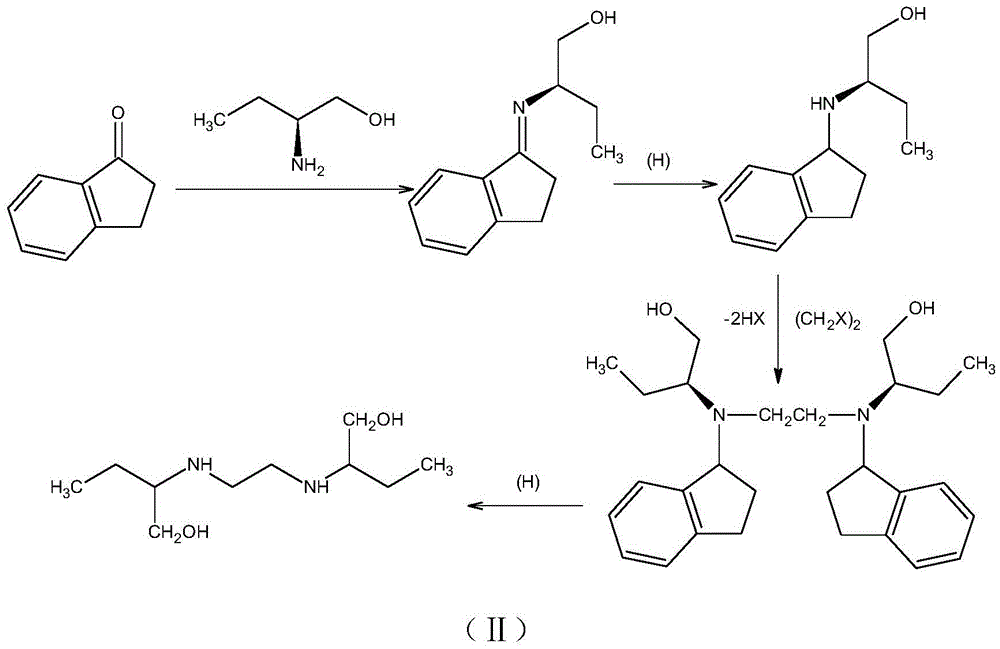
Method for synthesizing (S)-2-aminobutanol
A technology of aminobutyric acid and methylenebisacrylamide, which is applied in the field of synthesis of -2-aminobutanol, can solve problems such as inability to apply industrialized production, difficulty in obtaining raw materials, low yield and low efficiency, and the like, and achieve catalytic efficiency Improve, improve quality and yield, reduce the effect of by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078] (1) Preparation of supported palladium catalyst
[0079] 100 parts by weight of acrylic acid (pretreated by distillation under reduced pressure), 10 parts by weight of water-soluble chitosan (deacetylation degree 90%, molecular weight is 3 * 10 5 , purchased from Zhejiang Golden Shell Pharmaceutical Co., Ltd.), 3 parts by weight of N,N-methylenebisacrylamide were dissolved in 1000mL deionized water, and ultrasonically oscillated to make the mixture uniform. Then, nitrogen gas was continuously introduced, and the temperature was raised to 60 ℃, under stirring, slowly add 1 part by weight of initiator (the initiator is potassium peroxodisulfate / sodium bisulfite with a molar ratio of 2:1), react for 2 hours to obtain a hydrogel, adjust the pH value to neutral Afterwards, it was washed with ethanol, dried at 50°C to constant weight and crushed to 80 mesh to obtain a catalyst carrier;
[0080] According to the ratio of the catalyst carrier to the palladium chloride aqueous ...
Embodiment 2~-5
[0091] (1) Preparation of supported palladium catalyst
[0092] Other steps are with embodiment 1, and difference only is the concentration and the consumption of palladium chloride aqueous solution, see following table 2:
[0093] Table 2: The different consumptions of palladium chloride in embodiment 2~5
[0094]
[0095] (2) Catalytic hydrogenation
[0096] Other steps are with embodiment 1, and difference only is that added catalyst type and consumption, catalyzer adopts the supported palladium catalyst prepared by the step (1) of above-mentioned embodiment 2~5 respectively, the content of palladium active component is the same in each embodiment. It is 0.1% of the weight of (S)-2-aminobutyric acid, based on which the amount of supported palladium catalyst used in each embodiment is calculated. Embodiment 1~5 hydrogenation reaction required time contrast, see following table 3:
[0097] Table 3: The time required for the hydrogenation reaction in Examples 1 to 5
[...
Embodiment 6
[0103] (1) Preparation of supported ruthenium catalyst
[0104] 100 parts by weight of acrylic acid (pretreated by distillation under reduced pressure), 10 parts by weight of water-soluble chitosan (deacetylation degree 90%, molecular weight is 3 * 10 5 , purchased from Zhejiang Golden Shell Pharmaceutical Co., Ltd.), 3 parts by weight of N,N-methylenebisacrylamide were dissolved in 1000mL deionized water, and ultrasonically oscillated to make the mixture uniform. Then, nitrogen gas was continuously introduced, and the temperature was raised to 60 ℃, under stirring, slowly add 1 part by weight of initiator (the initiator is potassium peroxodisulfate / sodium bisulfite with a molar ratio of 2:1), react for 2 hours to obtain a hydrogel, adjust the pH value to neutral Afterwards, it was washed with ethanol, dried at 50°C to constant weight and crushed to 80 mesh to obtain a catalyst carrier;
[0105] According to the ratio of the catalyst carrier to the ruthenium chloride aqueous ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com