Ascorbate glucoside crystalline powder and preparation method thereof
A technology of ascorbyl glucoside and crystalline powder, which is applied in the field of ascorbyl glucoside crystalline powder and its manufacturing, which can solve the problems of production cost consumption, light transmittance defects, low conversion rate, etc., reduce waste water discharge, ensure good quality, improve The effect of light transmittance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] A kind of manufacture method of ascorbyl glucoside crystalline powder, comprises the steps:
[0045] one, convert
[0046] Feeding: Add excessive L-ascorbic acid or L-ascorbic acid and its salt, β-cyclodextrin, and the mass ratio of L-ascorbic acid or L-ascorbic acid and its salt to β-cyclodextrin is 10:12-16. Then add 2% to 10% of the substrate mass antioxidant (NaHSO 3 、Na 2 SO 3 、Na 2 S 2 o 5 any one), adjust the pH to 4.5 to 5.5, feed nitrogen with a purity of ≥99% at 35°C to 45°C, add cyclodextrin glucosyltransferase, and stir for 20 to 26 hours until the concentration of ascorbyl glucoside is The conversion stops at 120g / L~140g / L.
[0047] Hydrolysis: Then raise the temperature to 42-50°C, add glucoamylase and stir for 3-4 hours.
[0048] Removal of residual reducing sugar: then adjust the pH to 5.0-6.0 at a temperature of 30-40°C, add the yeast, stir and activate with air, then stop the air and continue stirring for 8-15 hours.
[0049] Heat sterilizat...
Embodiment 2
[0065] A kind of manufacture method of ascorbyl glucoside crystalline powder, its step is basically the same as embodiment 1, but conversion step is specifically as follows:
[0066] 1. Feeding
[0067] 1) Turn on the air compressor, nitrogen generator and its inlet and outlet valves successively. Make sure N 2 Purity ≥ 99.95%, nitrogen generator outlet N 2 The flow rate is controlled at 1.2~1.4N·m 3 / h. Lead the nitrogen line into the conversion tank.
[0068] 2) Inject 10M water into the conversion tank 3 . Turn on the stirring and raise the temperature of the water to 40°C.
[0069] 3) Put in 1800kgVC. Rinse the VC residue stuck to the tank wall with water. Use 30-40w / v% NaOH to adjust the pH to about 4.8; if VCNa is used as the substrate, it can be added in equimolar amounts, and a small amount of L-ascorbic acid and L-sodium ascorbate (mass ratio of about 1 : 6), make the pH of the conversion system around 4.8.
[0070] 4) Put in 2800kg of β-cyclodextrin.
[007...
Embodiment 37m3
[0084] Example 37m 3 Purification process of conversion liquid
[0085] A kind of manufacture method of ascorbyl glucoside crystalline powder, its step is basically the same as embodiment 1, but purification step is specifically as follows:
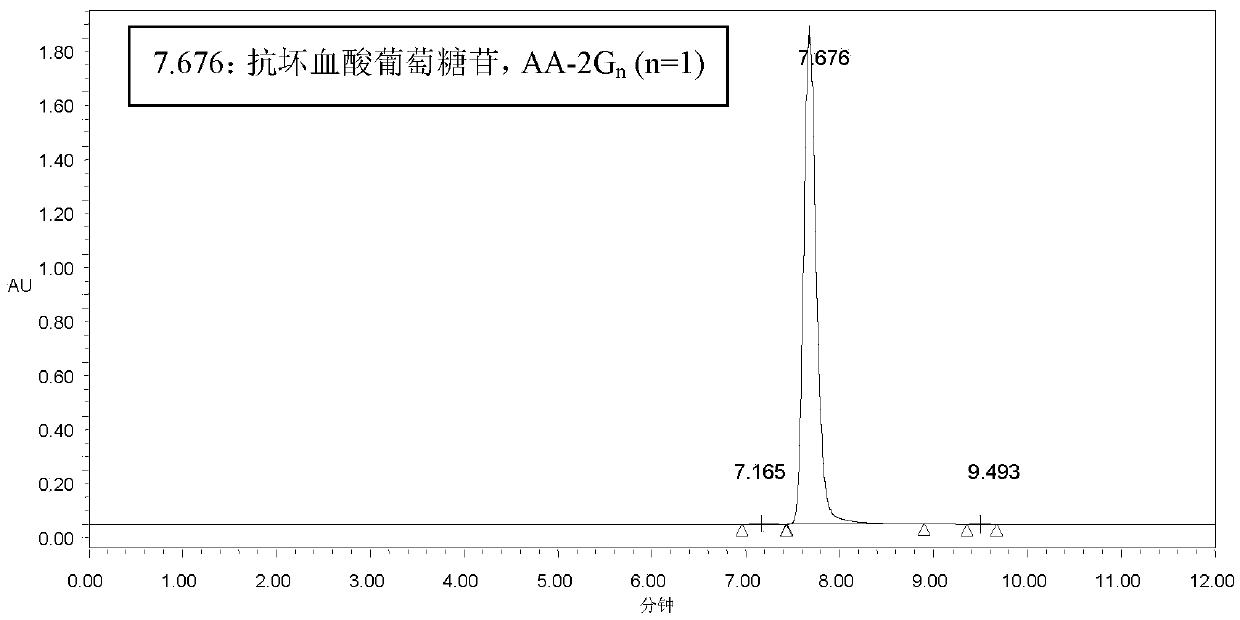
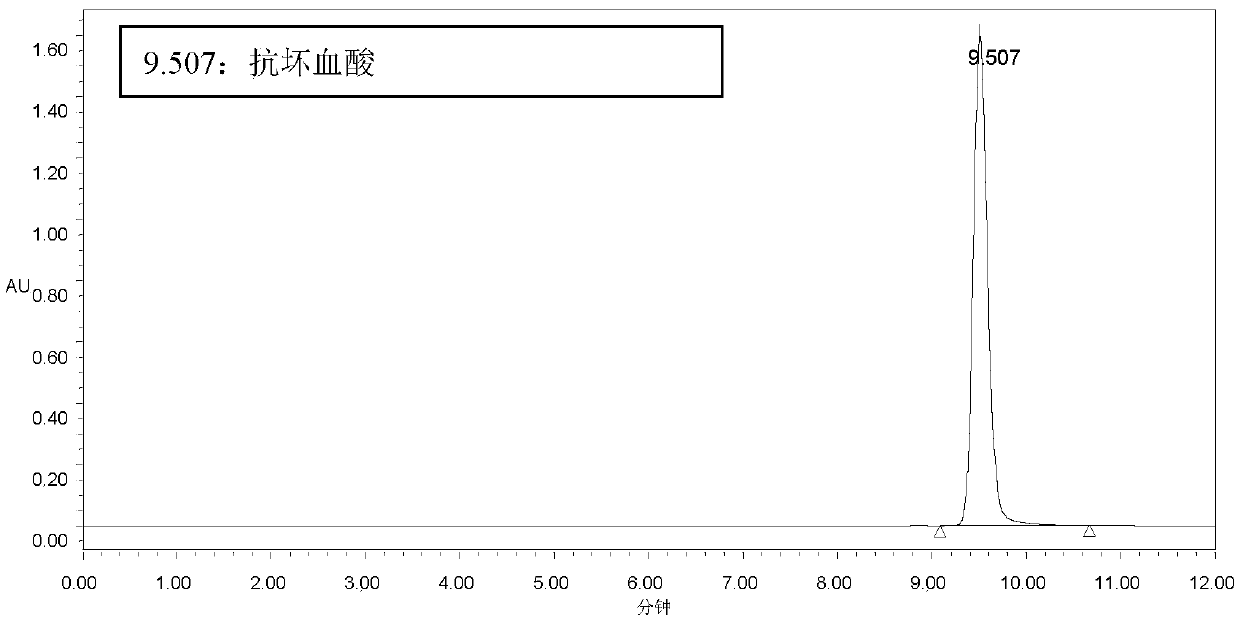
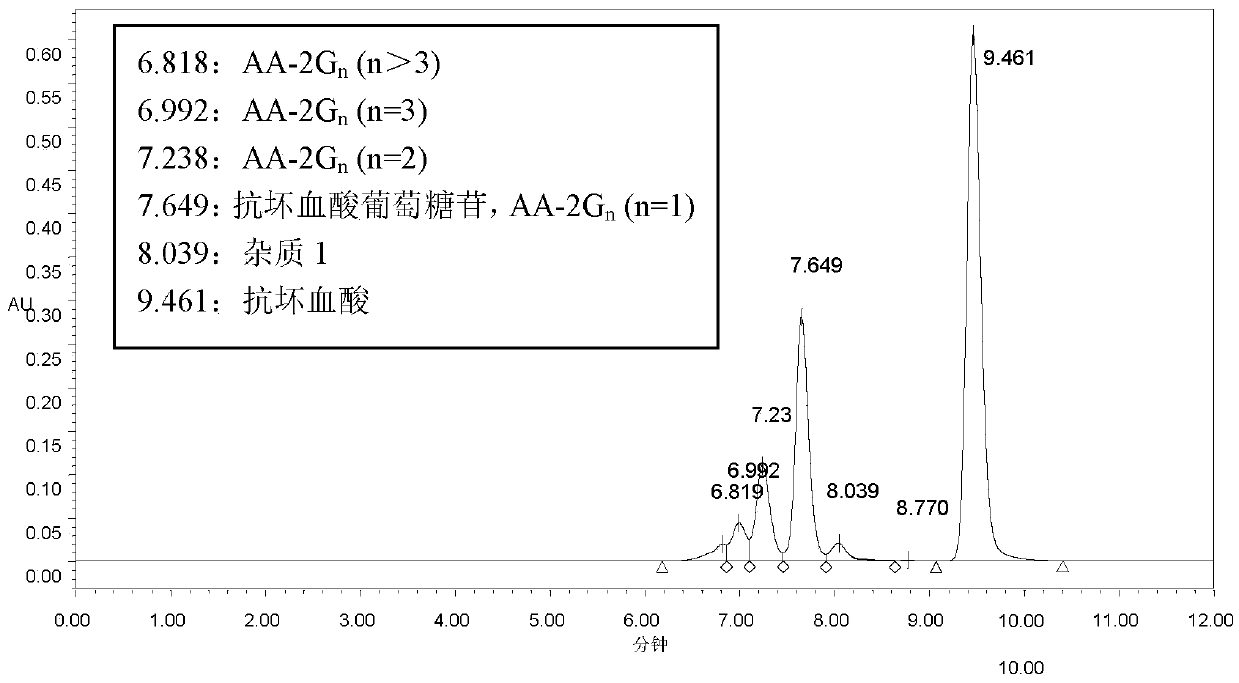
[0086] 7m 3 The substrate dosage of the transformation system for transformation is: β-cyclodextrin 1400kg, VC 900kg. After conversion, ascorbyl glucoside 165.2g / L was obtained, and remaining VC was 34.8g / L. According to the amount of ascorbyl glucoside generated, the theoretical utilization of VC in the conversion was calculated to be 601kg. Therefore, the theoretical utilization rate of VC was 67%. The actual total remaining VC is 243.6kg.
[0087] 1. Microfiltration system
[0088] Microfiltration membrane model: PL-M-132, manufacturer: Anhui Pulang Membrane Technology Co., Ltd.
[0089] The microfiltration system is used to remove impurities such as bacteria and miscellaneous proteins. The microfiltration process adds about 12m o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Relative molecular mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com