Application of a nitrogen heterocyclic compound functionalized ion exchange material in recovery of rhenium in wastewater
A technology of ion exchange materials and nitrogen heterocyclic compounds, which is applied in the field of resource recovery in industrial waste liquids, can solve the problems of high price of extraction agents, high requirements for operation skills, and time-consuming, and achieves that the adsorption effect is not attenuated obviously, and the preparation method The process is simple, and the effect of overcoming the complexity of the synthesis process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
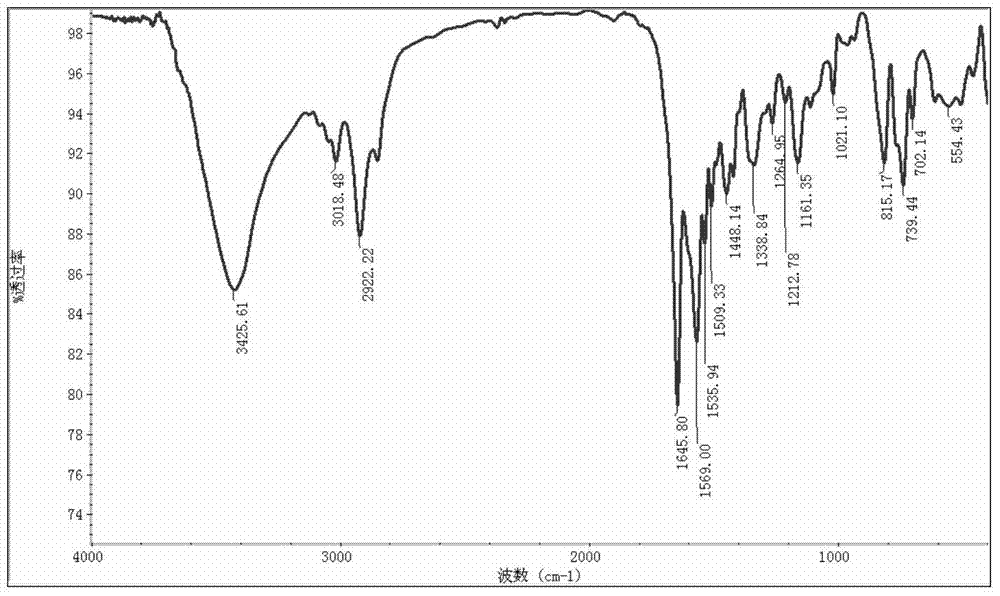
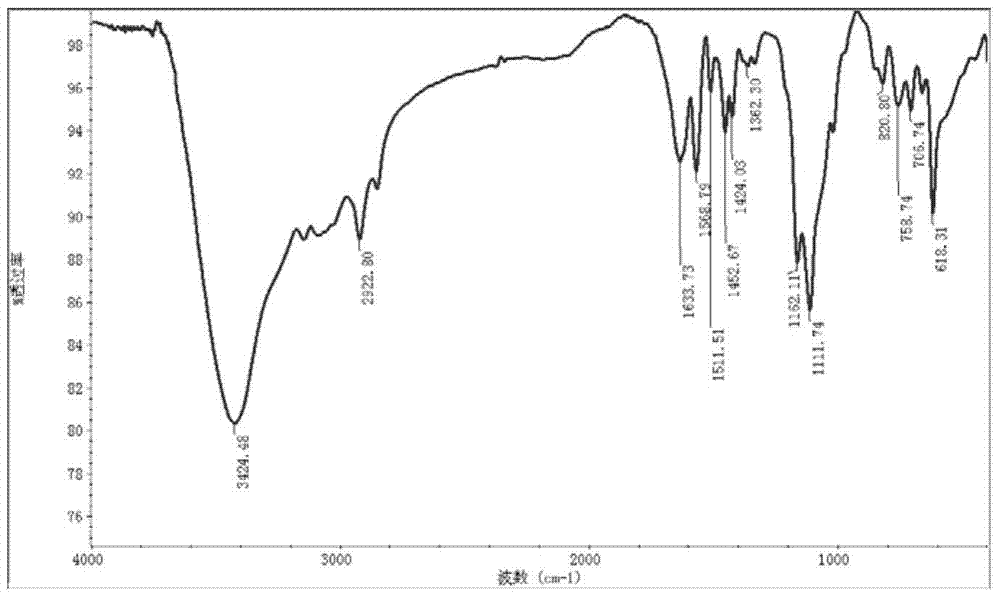
Image
Examples
Embodiment 1
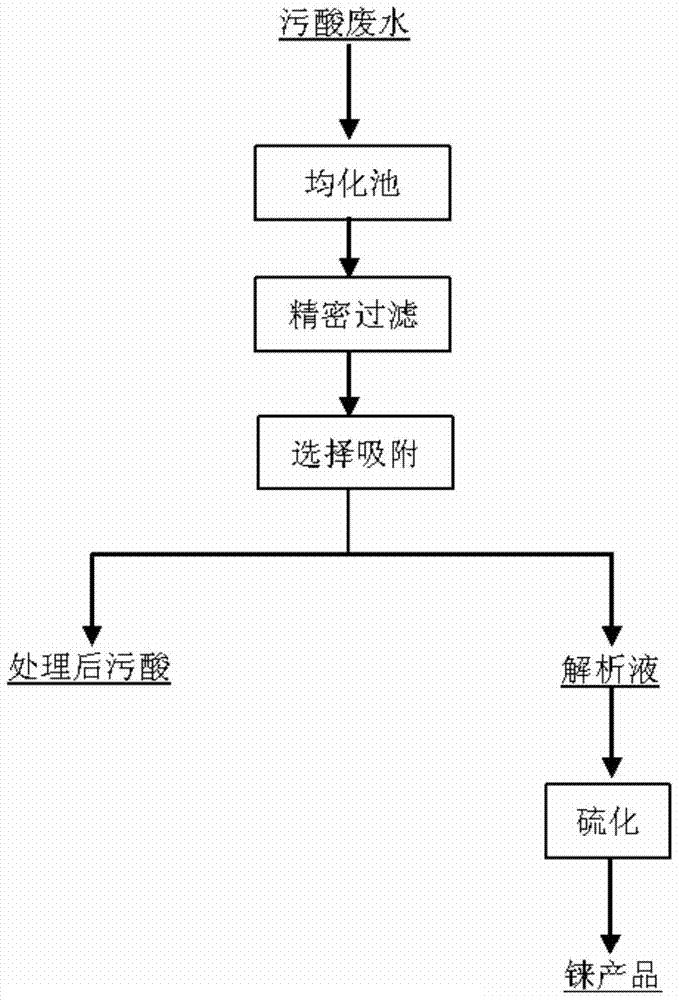
[0038] (1) Preparation of adsorption material
[0039]a. carry out chloromethylation to polypropylene-styrene fiber, take chloromethyl methyl ether as chloromethyl source, Lewis acid, sulfuric acid, hydrochloric acid are catalyst, the molar ratio of Lewis acid and raw material is 1.1:1, sulfuric acid The mass fraction is 80%, V hydrochloric acid: V sulfuric acid is 2:1, the reaction temperature is 50°C, and the reaction time is 12h; b. After separating the reaction product of a, add a solution with a mass fraction of α-aminopyridine to react to control the reaction The temperature is 60°C, and the reaction time is 24h; c. Finally, the reaction product is dried to a constant weight at a temperature of 50-70°C to obtain a nitrogen heterocyclic compound functionalized ion exchange material; d. Use 2mol / L sulfuric acid The sodium solution was transformed at 25°C for 1 hour before use.
[0040] (2) Application of adsorption materials
[0041] a After the rhenium-containing pollut...
Embodiment 2
[0045] (1) Preparation of adsorption material
[0046] a. Carry out chloromethylation to styrene-divinylbenzene pellet, take chloromethyl methyl ether as chloromethyl source, Lewis acid, sulfuric acid, hydrochloric acid are catalyst, the molar ratio of Lewis acid and raw material is 1.1:1 , the mass fraction of sulfuric acid is 80%, V hydrochloric acid: V sulfuric acid is 2:1, the reaction temperature is 50°C, and the reaction time is 12h; b. After separating the reaction product of a, add N-methylimidazole mass fraction to the solution of 8% for reaction , the reaction temperature is controlled at 60°C, and the reaction time is 24h; c. Finally, the reaction product is dried to a constant weight at a temperature of 50-70°C to obtain a nitrogen heterocyclic compound functionalized ion exchange material; d. L of sodium sulfate solution was transformed at 25°C for 1 hour before use.
[0047] (2) Application of adsorption materials
[0048] a After the rhenium-containing pollute...
Embodiment 3
[0052] (1) Preparation of adsorption material
[0053] a. Styrene-divinylbenzene film is carried out chloromethylation, take chloromethyl methyl ether as chloromethyl source, Lewis acid, sulfuric acid, hydrochloric acid are catalyst, the mol ratio of Lewis acid and raw material is 1.1:1, Sulfuric acid mass fraction is 80%, V hydrochloric acid: V sulfuric acid is 2:1, reaction temperature is 50 ℃, reaction time is 12h; The reaction temperature is 60°C, and the reaction time is 24h; c. Finally, the reaction product is dried to a constant weight at a temperature of 50-70°C to obtain a nitrogen heterocyclic compound functionalized ion exchange material; d. Use 2mol / L The sodium sulfate solution was transformed at 25°C for 1 hour before use.
[0054] (2) Application of adsorption materials
[0055] a After the rhenium-containing polluted acid wastewater is separated from the solid and liquid by free sedimentation. b Control the flow rate to be 8BV / h into the selective adsorption...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com