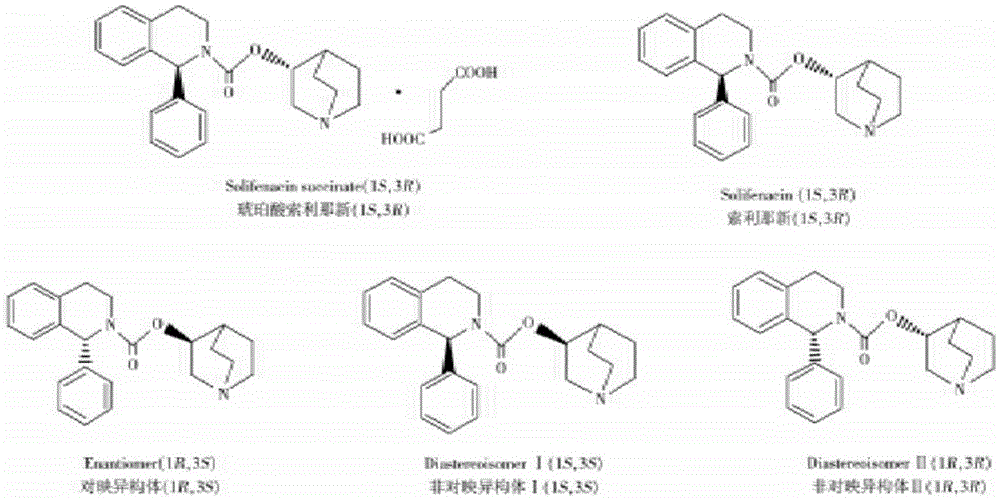
After-treatment for solifenacin and method for preparing succinic acid solifenacin
A technology of solifenacin and strong acid, applied in the field of preparing solifenacin succinate, can solve problems such as being unfavorable to operation and recycling, affecting salt formation and precipitation of crystals, affecting the purity of finished products, etc., and achieving easy operation and recycling. , the effect of reducing residual solvents and impurities, and simplifying the operation of post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1: Preparation of solifenacin by one-pot method
[0028] Add (R)-(-)-3-quinine alcohol (381g) and N,N-dimethylformamide (1.52L) into a 10L reactor, protect with nitrogen, stir at 30°C to dissolve; Bis(4-nitrophenyl) carbonate (1090 g) was added at 30°C. During the addition, the color of the solution turned into bright brownish yellow. After the addition was completed, the temperature was controlled at 30°C and reacted for 3 hours. TLC monitors that the raw material (R)-(-)-3-quinine alcohol reacts completely, and the raw material (R)-(-)-3-quinine alcohol is completely consumed.
[0029] Add (S)-1-phenyl-1,2,3,4-tetrahydroisoquinoline (816 g) in batches to the first step reaction system, control the temperature at 30°C, and react for 3 hours. TLC monitors the reaction status of raw material (S)-1-phenyl-1,2,3,4-tetrahydroisoquinoline and intermediates, such as (S)-1-phenyl-1,2,3,4-tetra If hydroisoquinoline disappears and intermediate I remains, (S)-1-phenyl-1...
Embodiment 2
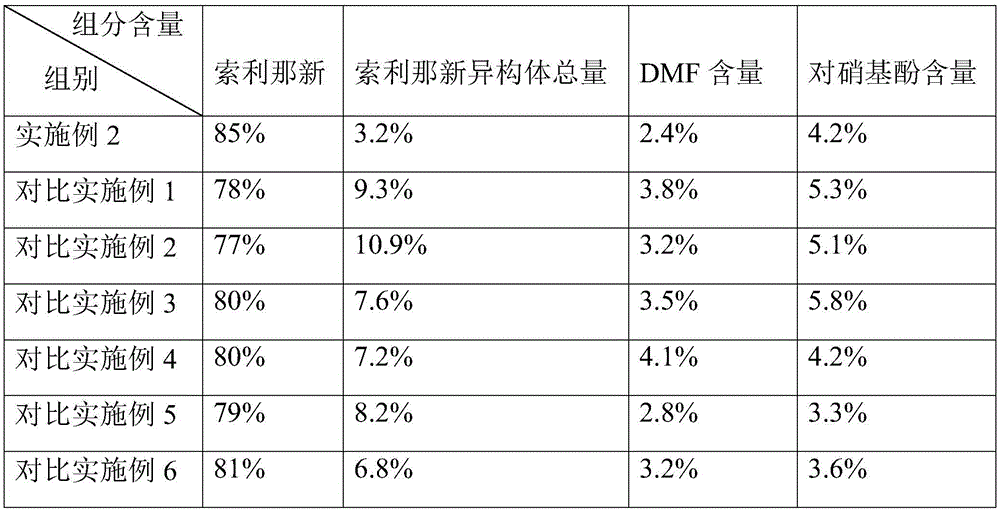
[0030] Example 2: Post-treatment of the total synthetic solution of solifenacin prepared by the one-pot method
[0031] Slowly add about 3.5L of dilute hydrochloric acid solution to the reaction kettle containing the synthetic total solution, the internal temperature rises during the dropping process, the dropping speed ensures that the temperature rise does not exceed 30°C, and the measured pH value is 1.7. Add 22L of water and 11L of isopropyl ether (about half the volume of water) to the reaction kettle; extract 3 times with isopropyl ether (the amount of water and isopropyl ether for each extraction is 2:1), and collect the extracted aqueous phase , add saturated sodium carbonate solution to adjust the pH value to 9, add dichloromethane and extract twice. The dichloromethane phase is washed 3 times with about an equal volume of water, and the dichloromethane phase is put into a plastic beaker to add sodium sulfate (the consumption of sodium sulfate is about 20% of the solv...
Embodiment 3
[0032] Embodiment 3: the preparation of solifenacin succinate
[0033] Add acetone to dissolve the oil obtained above; add succinic acid and stir at 60°C for 1 hour; stop heating and stirring and let it cool down to room temperature naturally; drop to 20°C and stir for about 2 hours to crystallize; suction filter to obtain a white solid, wash with acetone; Linacin-API crude product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com