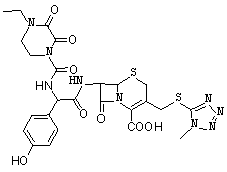
A kind of synthetic method of cefoperazone acid
A technology of cefoperazone acid and a synthesis method, applied in the field of medicine, can solve the problems of unstable acyl chloride intermediate, unfavorable industrial production, harsh reaction conditions and the like, and achieves the effects of simple preparation method, stable intermediate and improved yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
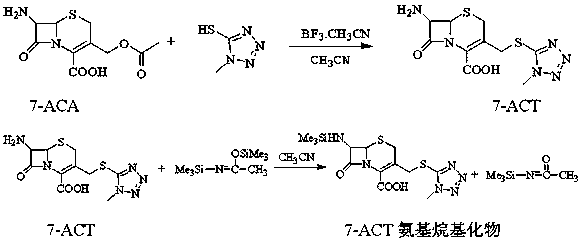
[0028] (1) Mix 65 g of boron trifluoride acetonitrile and 6.8 g of 1-methyl-5-mercaptotetrazolium, stir for 30 min, then add 14 g of 7-ACA, react at 30°C for 1 h, then transfer to 100 g Hydrolyze in purified water, add dilute ammonia water at 10°C until crystals appear, grow crystals for 30 minutes, continue to add dilute ammonia water to adjust pH to 3.0, grow crystals for 1 hour, filter with suction, wash with 60% acetone aqueous solution, and dry When the water content is ≤1%, 16.1 g of white solid 7-ACT is obtained with a purity of >99% and a mass yield of 115%;
[0029] (2) Mix 14 g of 7-ACT from step (1), 77 ml of acetonitrile, and 14 g of N,O-(bis)trimethylsilylacetamide, stir and dissolve until clear at a temperature of 25°C to obtain 7-ACT aminoalkylate solution, cooled to 0°C, set aside;
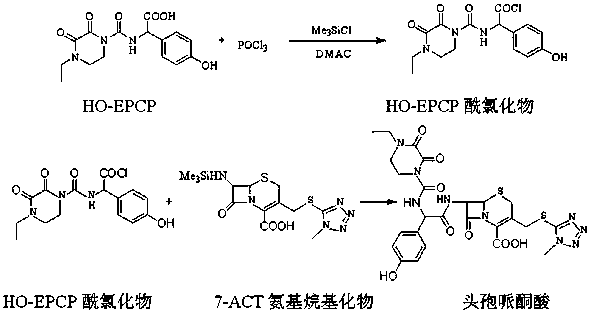
[0030] (3) Mix 15.5 g HO-EPCP, 45 ml N,N-dimethylacetamide, 25 ml acetonitrile and 8 g trimethylchlorosilane, stir to dissolve, cool down to -20°C, and then add 8.1 g trichlorosil...
Embodiment 2
[0033] (1) Mix 65 g of boron trifluoride acetonitrile and 6.8 g of 1-methyl-5-mercaptotetrazolium, stir for 30 min, then add 14 g of 7-ACA, react at 30°C for 1 h, then transfer to 100 g Hydrolyze in purified water, add dilute ammonia water at 10°C until crystals appear, grow crystals for 30 minutes, continue to add dilute ammonia water to adjust pH to 3.0, grow crystals for 1 hour, filter with suction, wash with 60% acetone aqueous solution, and dry When the water content is less than or equal to 1%, 7-ACT is obtained;
[0034] (2) Mix 14 g of 7-ACT from step (1), 90 ml of acetonitrile, and 14 g of N,O-(bis)trimethylsilylacetamide, stir and dissolve until clear at a temperature of 25°C to obtain 7-ACT aminoalkylate solution, cooled to 0°C, set aside;
[0035] (3) Mix 15 g HO-EPCP, 60 ml N,N-dimethylacetamide, 40 ml acetonitrile and 8 g trimethylchlorosilane, stir to dissolve, cool down to -20°C, and then add 8.1 g trichlorosilane Oxyphosphorus, heat preservation reaction, HP...
Embodiment 3
[0038] (1) Mix 65 g of boron trifluoride acetonitrile and 6.8 g of 1-methyl-5-mercaptotetrazolium, stir for 30 min, then add 14 g of 7-ACA, react at 30°C for 1 h, then transfer to 100 g Hydrolyze in purified water, add dilute ammonia water at 10°C until crystals appear, grow crystals for 30 minutes, continue to add dilute ammonia water to adjust pH to 3.0, grow crystals for 1 hour, filter with suction, wash with 60% acetone aqueous solution, and dry When the water content is less than or equal to 1%, 7-ACT is obtained;
[0039](2) Mix 13 g of 7-ACT from step (1), 70 ml of acetonitrile, and 13 g of N,O-(bis)trimethylsilylacetamide, stir and dissolve until clear at a temperature of 25°C to obtain 7-ACT aminoalkylate solution, cooled to 0°C, set aside;
[0040] (3) Mix 16 g HO-EPCP, 60 ml N,N-dimethylacetamide, 40 ml acetonitrile and 10 g trimethylchlorosilane, stir to dissolve, cool down to -25°C, and then add 7.5 g trichlorosilane Oxyphosphorus, heat preservation reaction, HP...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com