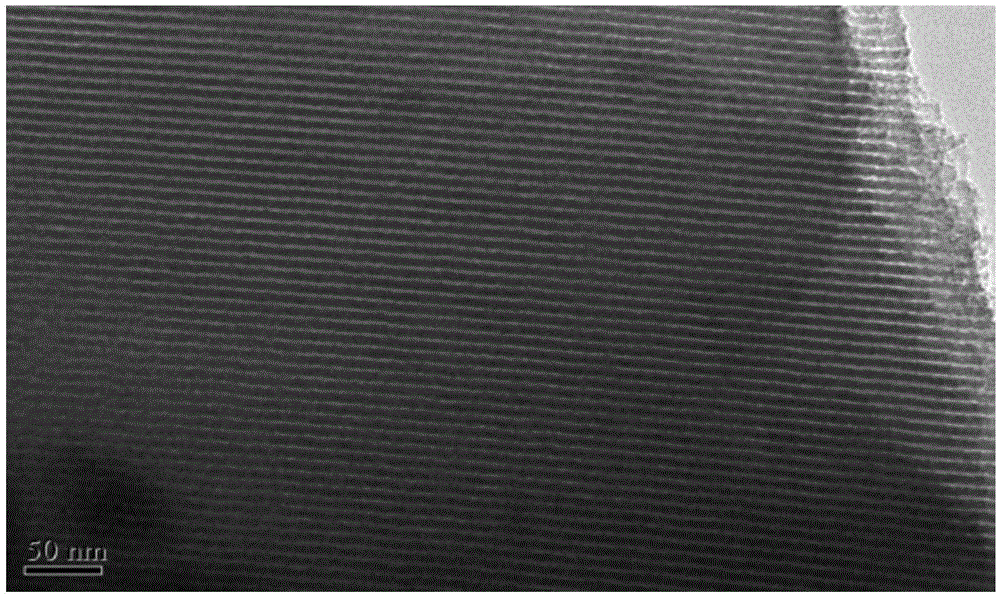
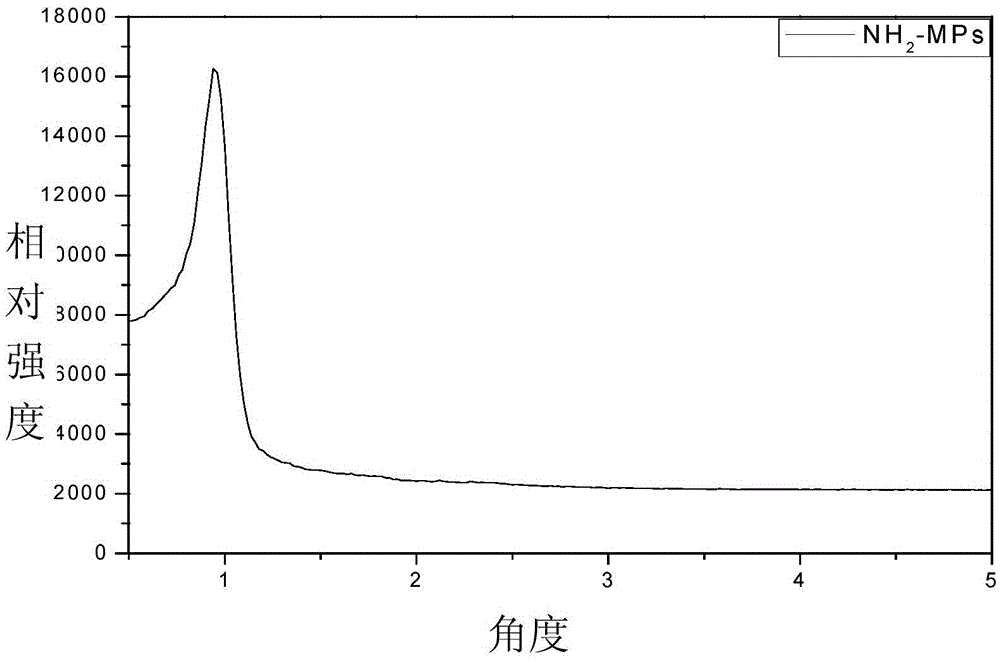
Amino-functionalization ordered mesopore phenolic resin material and preparing method thereof
A technology of amino functionalization and phenolic resin, applied in the field of phenolic resin materials, can solve the problems of high cost, low utilization rate of nitrogen source, harmful environment, etc., and achieve the effect of short synthesis cycle, large specific surface area and avoiding post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0018] Some embodiments of one aspect of the present invention provide a method for preparing an amino-functionalized ordered mesoporous phenolic resin material, which includes: using m-nitrophenol and formaldehyde as raw materials, synthesizing a soluble phenolic resin in an alkaline liquid phase environment The precursor is then mixed with the template agent in an organic solution, and the cured intermediate is obtained by the method of solvent volatilization and self-assembly, and finally the cured intermediate is placed in a protective atmosphere and roasted to remove the template agent, that is, the amino functionalized Ordered mesoporous phenolic resin materials.
[0019] In a preferred embodiment, the preparation method comprises the following steps:
[0020] (1) reacting m-nitrophenol and formaldehyde in an alkaline liquid phase system environment and at a temperature of 100-120°C for more than 1 hour to form a nitro-functionalized phenolic resin precursor,
[0021] (...
Embodiment 1
[0053] Step 1: Synthesis of nitro-functionalized polymer precursors: Add 1.127g m-nitrophenol to a three-necked flask equipped with a stirrer in an oil bath at 40°C, and then add 2.413ml of 35-40wt% formaldehyde solution, stirred for 30min to dissolve and mix well. Add saturated sodium hydroxide alcohol solution, adjust the pH between 8 and 9, stir for 30min, raise the temperature to 110°C, adjust the rotation speed to 350rpm, stir at constant temperature for 2h, cool to room temperature after the reaction, and then adjust with 2mol / L hydrochloric acid solution The pH of the reaction solution is 7. After concentrating and evaporating to remove water at 50° C., four times of absolute ethanol is added to dilute and dissolve according to the mass ratio of the reaction solution to obtain a soluble precursor of nitro-functionalized resole phenolic resin.
[0054] Step 2: Self-assembly of nitro-functionalization: Mix and stir 1gF127, a triblock copolymer template agent, and 20g abso...
Embodiment 2
[0057] Step 1: Synthesis of nitro-functionalized polymer precursors: Add 3.381g m-nitrophenol to a three-necked flask equipped with a stirrer in an oil bath at 40°C, and then add 7.239ml of 35-40wt% formaldehyde solution, stirred for 30min to dissolve and mix well. Add saturated sodium hydroxide alcohol solution, adjust the pH between 8 and 9, stir for 30min, raise the temperature to 110°C, adjust the rotation speed to 350rpm, stir at constant temperature for 2h, cool to room temperature after the reaction, and then adjust with 2mol / L hydrochloric acid solution The pH of the reaction solution is 7. After concentrating and evaporating to remove water at 50° C., four times of absolute ethanol is added to dilute and dissolve according to the mass ratio of the reaction solution to obtain a soluble nitro-functionalized resole phenolic resin precursor.
[0058] Step 2: Nitro-functionalized self-assembly: Mix and stir 1gF127, a triblock copolymer template agent, and 20g absolute etha...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com