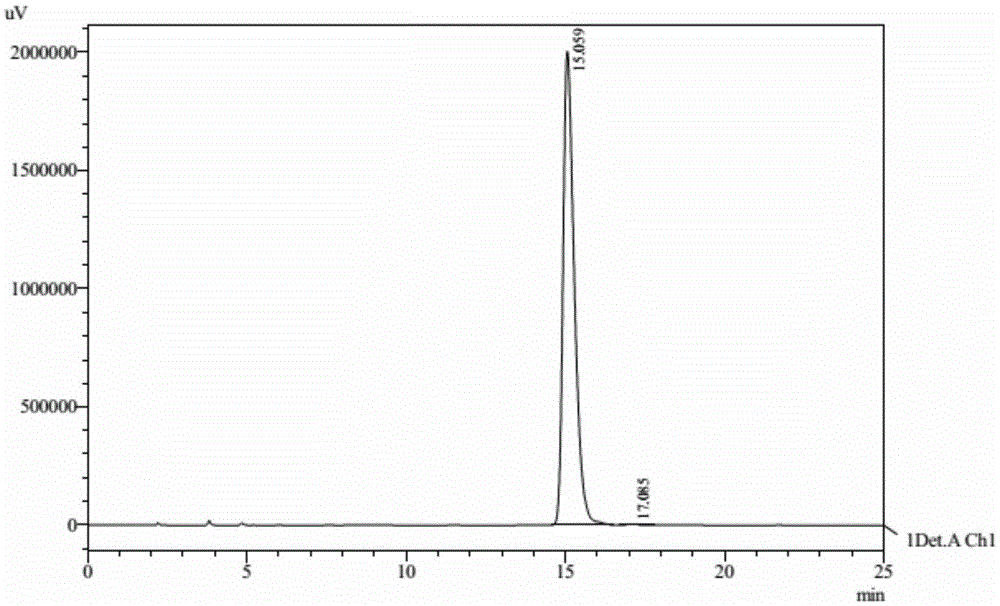
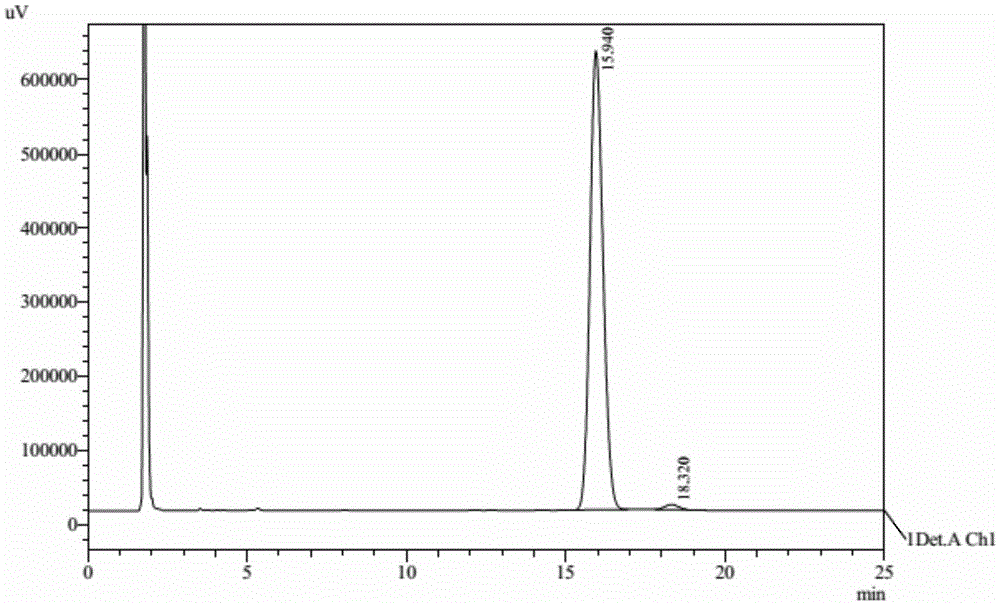
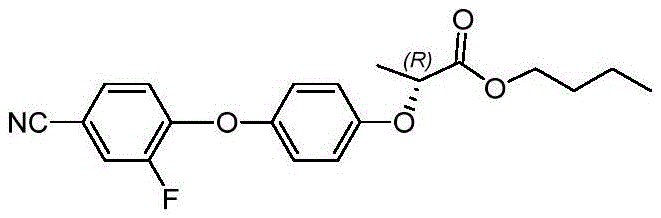
Preparation method of cyhalofop-butyl
A technology of cyhalofop-ethyl and diflubenil, which is applied in the field of preparation of cyhalofop-ethyl, can solve the problems of unrecoverable by-products of toluenesulfonic acid, racemization of the product configuration, and affecting product quality, etc., and achieve the reduction of three wastes Emissions, improved reaction selectivity, and environmental protection effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Embodiment 1 A kind of preparation method of cyhalofop-methyl, comprises the following steps:
[0038] 1) etherification reaction
[0039]Put 100mL of N,N-dimethylacetamide, 50g of potassium carbonate, 5g of triethylenediamine and 60.3g of phase transfer catalyst 18-crown into a 250mL four-necked flask, and then put (R)-2-(4 26g (0.14mol) of -hydroxyphenoxy)propionic acid, a large number of bubbles are produced; after (R)-2-(4-hydroxyphenoxy)propionic acid is fed, add 20g of 3,4-difluorobenzonitrile (0.14mol), then the temperature was raised to 60° C., and the reaction was incubated for 3 hours, and the reaction ended. Distill under reduced pressure to remove the solvent, drop to room temperature and add 150 mL of water to dissolve, adjust the pH value to 4-5 with 15% dilute hydrochloric acid, stir to precipitate a solid, and filter to obtain (R)-2-[4-(2-fluoro-4-carbonitrile base)-phenoxy]-propionic acid for subsequent use.
[0040] 2) Esterification reaction
[00...
Embodiment 2
[0043] Embodiment 2 A kind of preparation method of cyhalofop-methyl, comprises the following steps:
[0044] 1) etherification reaction
[0045] Put 100mL of N,N-dimethylacetamide, 40g of potassium carbonate, 0.3g of hexamethylenetetramine and 3g of phase transfer catalyst tetrabutylammonium bromide into a 250mL four-necked flask, and then put (R)- 26g (0.14mol) of 2-(4-hydroxyphenoxy)propionic acid, a large number of bubbles are produced; after the feeding of (R)-2-(4-hydroxyphenoxy)propionic acid is completed, add 3,4-di Fluorobenzonitrile 22g (0.16mol), then heated up to 90°C, kept the temperature for 2 hours, and the reaction ended. Distill under reduced pressure to remove the solvent, drop to room temperature and add 250mL of water to dissolve, adjust the pH value to 3-4 with 30wt.% dilute sulfuric acid, stir to precipitate a solid, filter to obtain (R)-2-[4-(2-fluoro-4- Nitrile)-phenoxy]-propionic acid for later use.
[0046] 2) Esterification reaction
[0047] In a...
Embodiment 3
[0049] Embodiment 3 A kind of preparation method of cyhalofop-methyl, comprises the following steps:
[0050] 1) etherification reaction
[0051] Put 100mL of N,N-dimethylacetamide, 40g of potassium carbonate, 1g of diazabicyclo and 0.5g of phase transfer catalyst benzyltriethylamine into a 250mL four-neck flask, and then put (R)-2- (4-hydroxyphenoxy) propionic acid 26g (0.14mol), there is a large amount of bubbles to produce; wait for (R)-2-(4-hydroxyphenoxy) propionic acid to feed in, then drop into 3,4-difluorobenzene 20 g (0.14 mol) of nitrile, and then the temperature was raised to 85° C., and the reaction was kept for 2 hours, and the reaction was completed. Remove the solvent by distillation under reduced pressure, add 250 mL of water to dissolve at room temperature, adjust the pH value to 3-4 with 30% dilute phosphoric acid, stir to precipitate a solid, and filter to obtain (R)-2-[4-(2-fluoro-4-carbonitrile base)-phenoxy]-propionic acid for subsequent use.
[0052] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com