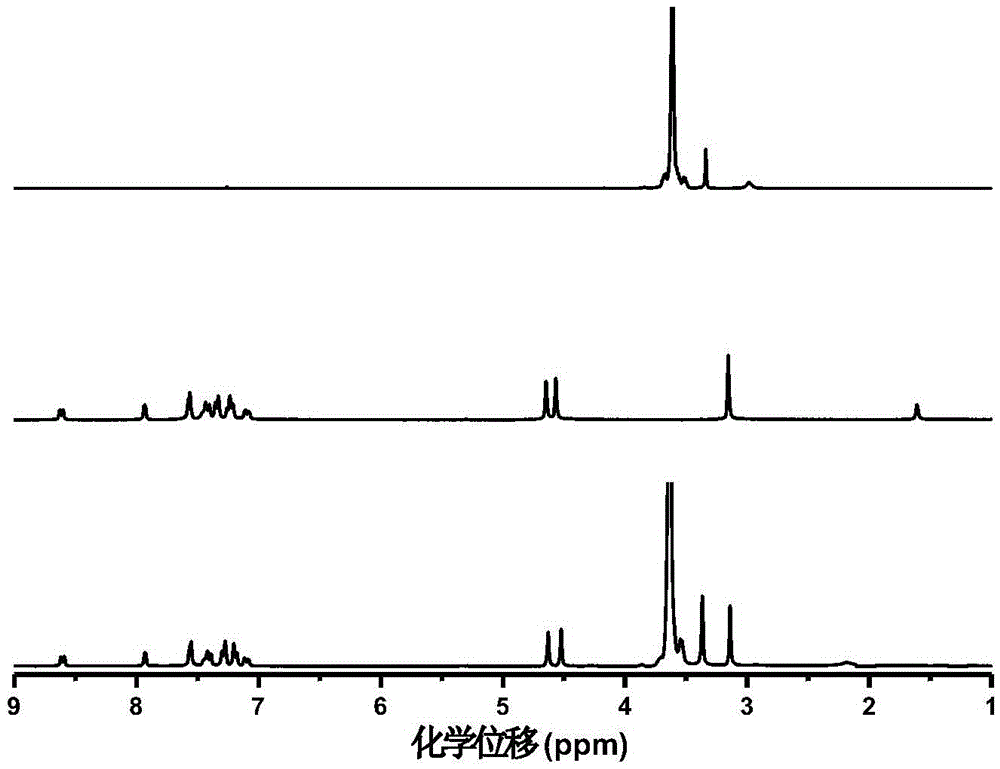
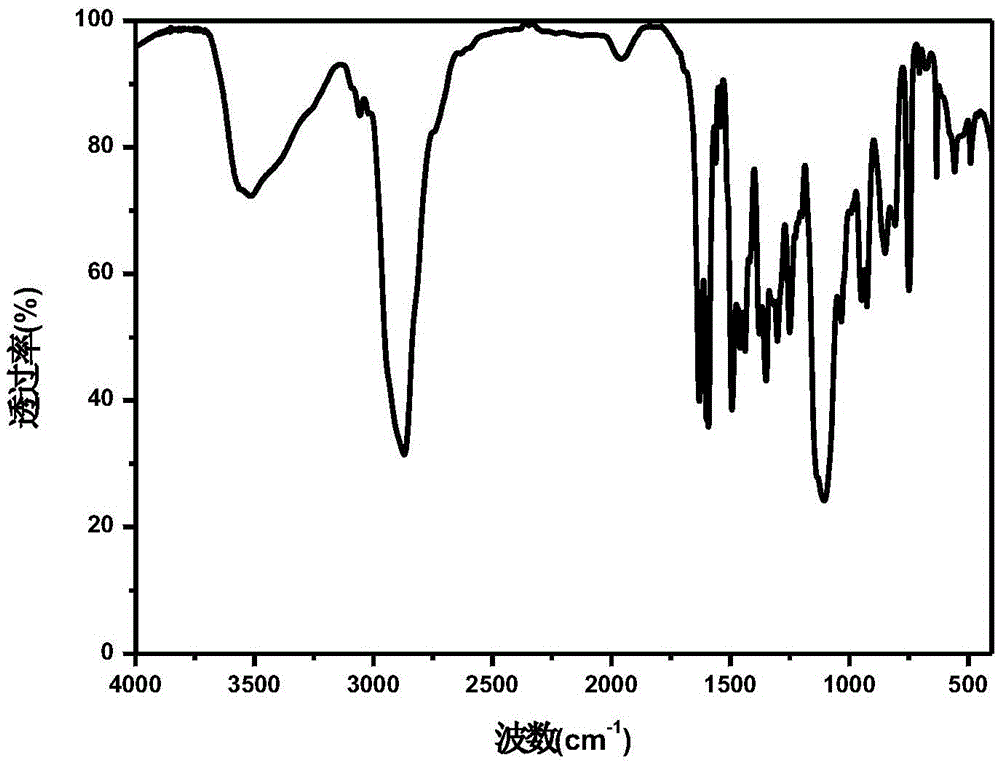
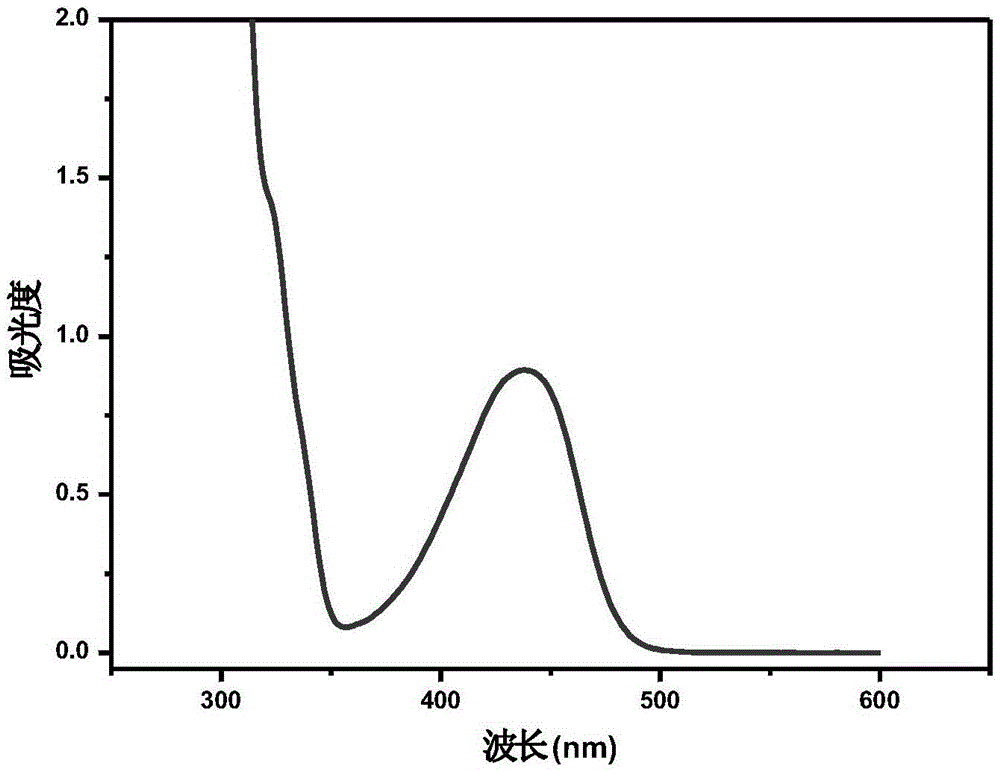
Water-soluble thiaxanthon-polyether visible light initiator and preparing method and application thereof
A thioxanthone, water-soluble technology, applied in the field of water-soluble thioxanthone-polyether visible light initiator and preparation, can solve the disadvantageous advantages of macromolecular initiators, complex synthesis methods, hindering photoactive groups, etc. problem, to achieve the effect of improving the light initiation efficiency, strong penetrating ability, and high initiation efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] (1) Preparation of thioxanthone:
[0035] In a 250mL single-necked round bottom flask, add 11.35g (0.05mol) of N-(4-bromophenyl)-N-methylacetamide, 7.70g (0.05mol) of thiosalicylic acid, 0.95g of copper powder, iodine Cuprous chloride 1.00g, anhydrous sodium carbonate 10.60g (0.10mol), N,N-dimethylformamide 100mL, heated to 100°C for 10h under stirring, cooled, filtered, the filtrate was concentrated, washed with water, and filtered. The filtrate was acidified with dilute hydrochloric acid to precipitate a white precipitate, filtered, and dried; weighed 9.03g into a 500mL single-necked flask, then added 500g of polyphosphoric acid (PPA), heated to 130°C and stirred for 5h, then heated the The reaction solution was poured into a beaker containing 700mL of ice water, cooled, and a yellow solid was precipitated, filtered, and the filter cake was washed with KHCO 3 The aqueous solution was washed, dried, and recrystallized in ethyl acetate: petroleum ether = 1:1 to obtain ...
Embodiment 2
[0045] (1) preparation of thioxanthone
[0046] Add N-(4-bromo-2-methylphenyl)-N-isopropylacetamide 13.45g (0.05mol) and thiosalicylic acid 7.70g (0.05mol) in a 250mL single-necked round bottom flask, Copper powder 0.95g, cuprous iodide 1.00g, anhydrous potassium carbonate 13.8g (0.10mol), N,N-dimethylacetamide 100mL, stirred at 160°C for 15h, cooled, filtered, the filtrate was concentrated, washed with water, filter. The filtrate was acidified with dilute hydrochloric acid to precipitate a white precipitate, filtered, and dried; weigh 10.29g into a 500ml one-necked flask, add 100mL of concentrated sulfuric acid under stirring, stir at room temperature for 1h, heat up to 60°C, react at 60°C for 2h, cool, Slowly added to 1L of boiling water; cooled to room temperature, filtered, the filter cake was washed with saturated NaOH aqueous solution, washed with water, dried, and subjected to silica gel column chromatography to obtain yellow 3-methyl-2-isopropylaminothioxanthone 6.98 ...
Embodiment 3
[0056] (1) preparation of thioxanthone
[0057] Add 16.25 g (0.05 mol) of N-(4-bromo-2-ethylphenyl)-N-hexylacetamide, 7.70 g (0.05 mol) of thiosalicylic acid, and copper powder into a 250 mL single-necked round bottom flask 0.95g, cuprous iodide 1.00g, sodium hydroxide 4.0g (0.10mol), N,N-dimethylformamide 100mL, stirred and heated to reflux for 18h, cooled, filtered, the filtrate was concentrated, washed with water, filtered. The filtrate was acidified with dilute hydrochloric acid to precipitate a white precipitate, filtered, and dried; weighed 11.97g into a 500ml single-necked flask, added 100mL of concentrated sulfuric acid under stirring, stirred at room temperature for 1h, raised the temperature to 80°C, reacted at 80°C for 10h, cooled, Slowly added to 1L of boiling water; cooled to room temperature, filtered, the filter cake was washed with saturated KOH aqueous solution, washed with water, dried, and subjected to silica gel column chromatography to obtain 9.26 g of yel...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com