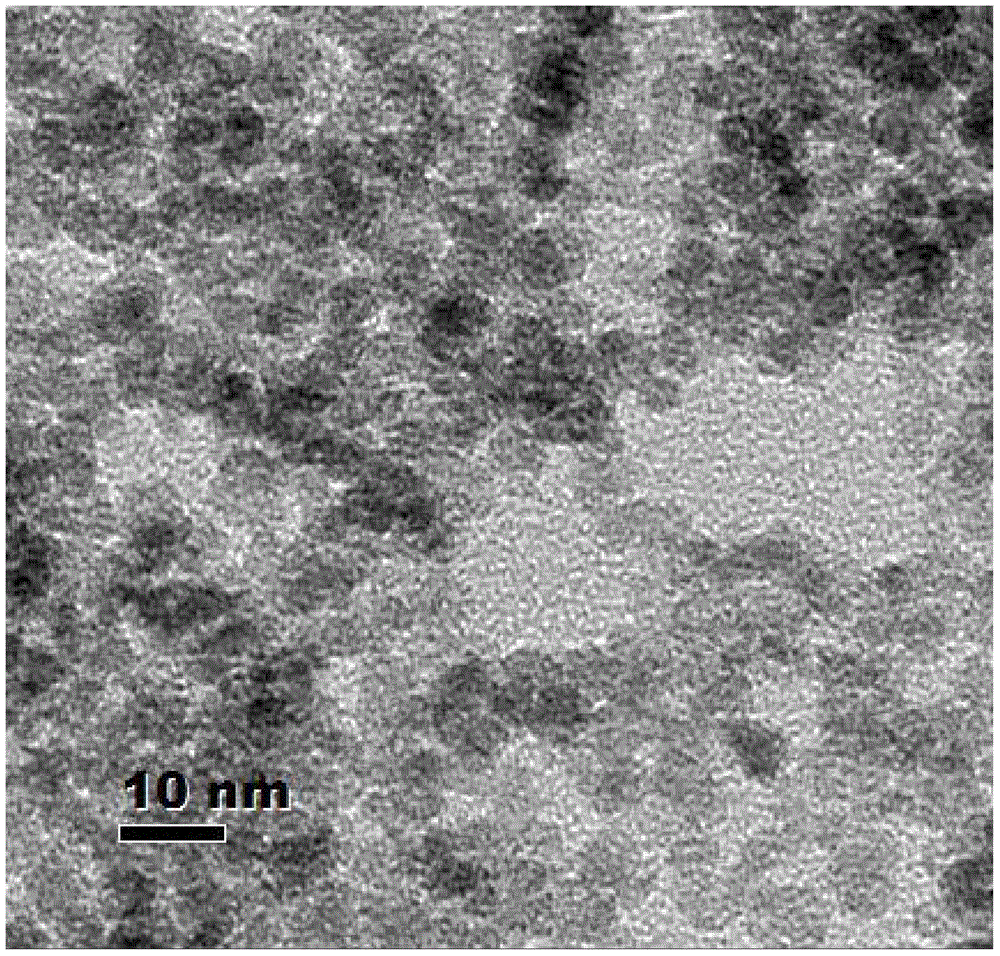
Reduced carboxy alkyl dextriferron injection and preparation method thereof
A technology of iron dextran and carboxyalkyl, which is applied in the field of injection containing reduced carboxyalkyl iron dextran and its preparation, and can solve iron dextran allergic reactions, multiple administration times, and long administration time, etc. problems, achieve low immunogenicity, good effect, and reduce the number of administrations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0098] The preparation of embodiment 1 sorbitol carboxyalkyl ether dextran iron particles
[0099] Preparation of sorbitol carboxyalkyl ether dextran
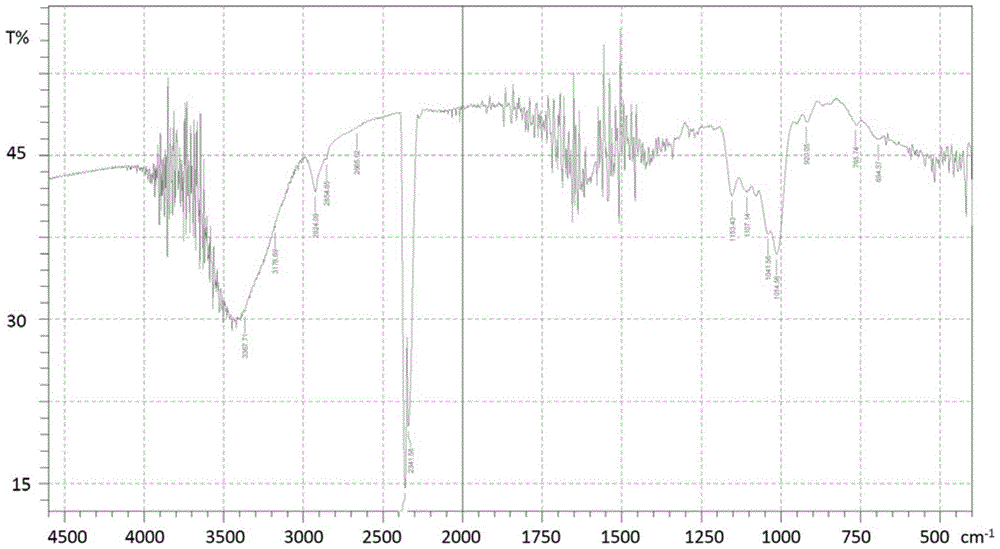
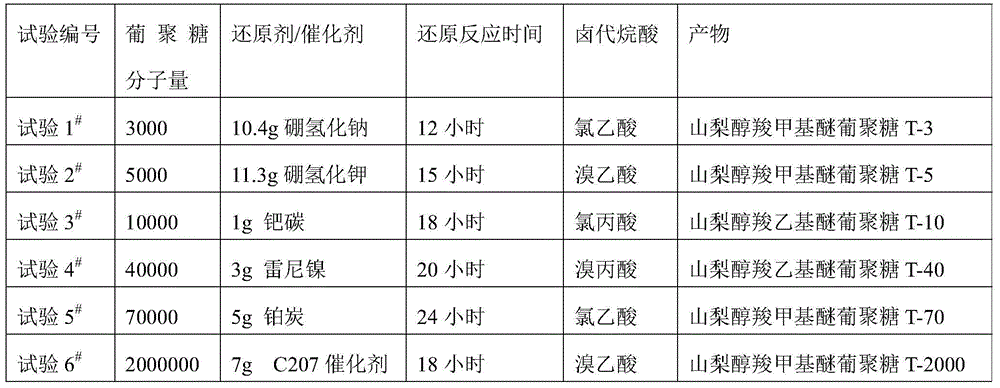
[0100]The dextran of 50g is dissolved in the purified water of 50g, after stirring it to dissolve completely, add 20g concentration and be the NaOH solution of 50% and the sodium borohydride of 10.4g (test 1 # ) or 11.3g potassium borohydride (test 2 # ) for reduction or catalytic hydrogenation (Test 3 # ~6 # ) for reduction, see Table 1 for details, react at room temperature, then add 34g concentration of 50% NaOH solution and halogenated alkanoic acid such as 27.6g chloroacetic acid or 31.8g bromoacetic acid to carry out carboxyalkylation reaction, after 12h, add 4M hydrochloric acid The reaction was terminated at pH 7.0, and a white product was obtained by precipitation. test 1 # The infrared spectrum of the reaction product is shown in figure 2 As shown, it conforms to the infrared spectrum of sorbitol carboxymethy...
Embodiment 2
[0108] The preparation of embodiment 2 sorbitol carboxyalkyl ether dextran iron injection
[0109] Test 1 in embodiment 1 # ~Experiment 6 # The prepared sorbitol carboxyalkyl ether dextran iron was dissolved in an appropriate amount of water for injection according to the prescription amount shown in Table 3. Add 0.05-0.2% (w / v) activated carbon, adjust the pH value of the solution to 5.5-6.5 with 1M hydrochloric acid solution under stirring, and then keep the drug solution at 60-70°C for 20-30 minutes. Then use 1M sodium hydroxide solution to adjust the pH value of the solution to 10.0-11.5, continue to keep the medicinal solution at a temperature of 60-70° C. for 10-15 minutes, and then filter and decarbonize. Add water for injection to the full amount of the prescription, check the pH value of the solution, and adjust the pH value of the solution to the range of 10.0 to 11.5 with an acid-base regulator if necessary; filter the liquid medicine with 0.4 μm and 0.22 μm micro...
Embodiment 3
[0112] Embodiment 3 test
[0113] Visible foreign object detection:
[0114] Injection 1 # ~ Injection 6 # After cold and heat cycle treatment, according to the second part of the Pharmacopoeia of the People's Republic of China (in this application, it can be referred to as "the second part of the Chinese Pharmacopoeia 2010" or similar abbreviation) appendix IXH of the first method (light inspection) in the foreign matter inspection method of the 2010 edition method) (this method may also be referred to as "light inspection method" or similar abbreviation in this application), and also inspect superparamagnetic iron oxide (Ferumoxytol), high-molecular-weight iron dextran, low-molecular-weight iron dextran, sucrose Iron and sodium ferric gluconate were tested, and the detection rate of visible foreign matter is shown in Table 4. Compared with existing products, the obtained embodiment of the present application has significantly lower detection rate of visible foreign matt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com