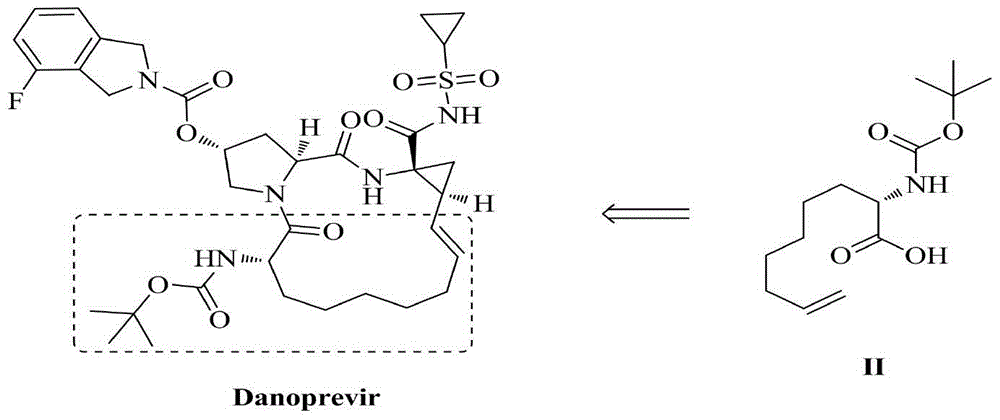
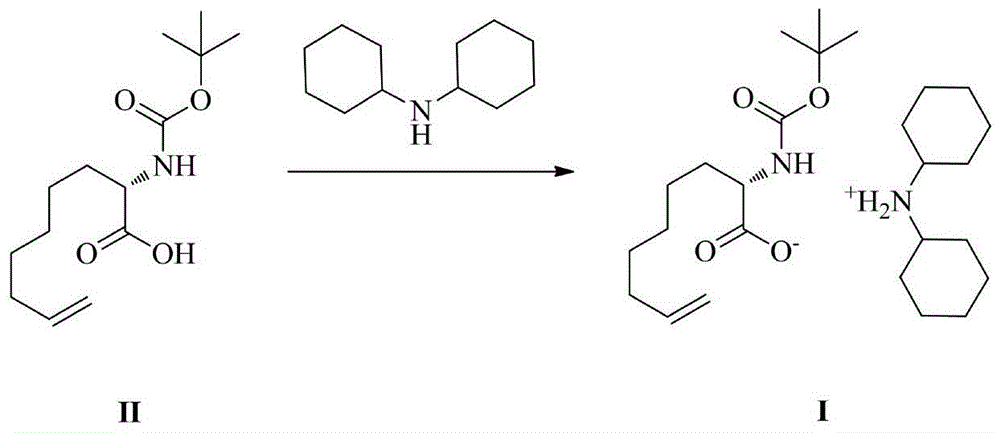
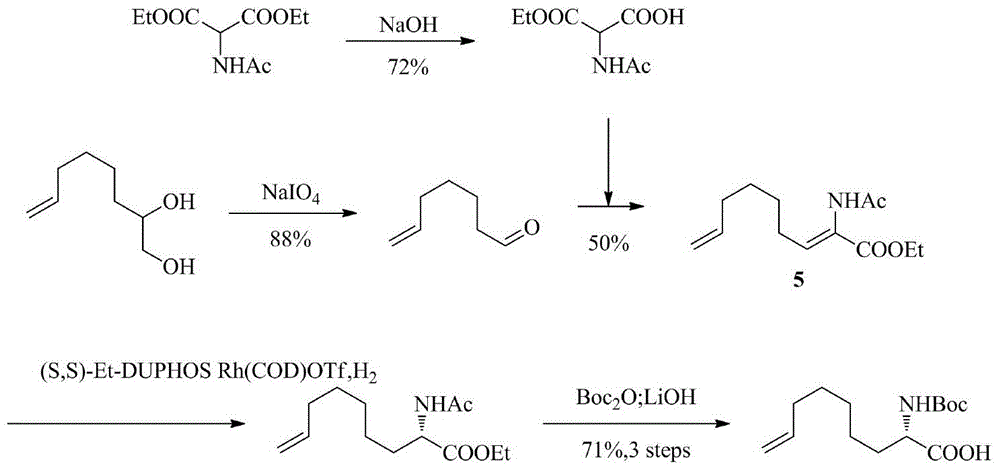
Method for synthesizing optically active intermediate N-tert-butoxycarbonyl-2-amino-8-nonenoic dicyclohexylamine salt
A technology of nonenoic acid dicyclohexylamine salt and tert-butoxycarbonyl, which is applied in the field of medicine, can solve the problems of expensive raw materials, cumbersome purification, and high cost, and achieve the effects of mild reaction conditions, short synthetic routes, and good product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Embodiment 1: a kind of synthetic method of optically active intermediate N-tert-butoxycarbonyl-2-amino-8-nonenoic acid dicyclohexylamine salt, specifically comprises the following steps:
[0051] S1. generate intermediate IV, which comprises the following substeps:
[0052] S11. Alkylation: Add methyl acetamidomalonate and 7-bromo-1-heptene to tetrahydrofuran, then add sodium iodide and sodium hydroxide to react at 0°C to obtain an alkylated product The molar mass ratio of methyl acetamidomalonate, 7-bromo-1-heptene and sodium iodide is 1:0.8:0.1; the molar mass ratio of methyl acetamidomalonate and sodium hydroxide is 1: 1;
[0053] S12. Hydrolyzing the alkylation product: performing a hydrolysis reaction on the alkylation product obtained in step S11 in a sodium hydroxide solution with a pH value of 12 at 0°C;
[0054] S13. Decarboxylation: add a hydrochloric acid solution with a pH value of 3 to the hydrolyzed solution, and perform a decarboxylation reaction at a ...
Embodiment 2
[0058] Embodiment 2: a kind of synthetic method of optically active intermediate N-tert-butoxycarbonyl-2-amino-8-nonenoic acid dicyclohexylamine salt, specifically comprises the following steps:
[0059] S1. generate intermediate IV, which comprises the following substeps:
[0060] S11. Alkylation: Add ethyl acetamidomalonate and 7-chloro-1-heptene to N,N-dimethylformamide and N,N-dimethylacetamide solvent, then add potassium iodide in Under the alkaline condition of 150 ℃, react to obtain the alkylation product; the alkali is potassium hydroxide and potassium carbonate; the molar mass ratio of ethyl acetamidomalonate, 7-chloro-1-heptene and potassium iodide is 1:2:0.5; the molar mass ratio of ethyl acetamidomalonate to base is 1:4;
[0061] S12. Hydrolyzing the alkylation product: performing a hydrolysis reaction on the alkylation product obtained in step S11 in a sodium hydroxide solution with a pH value of 10 at 100°C;
[0062] S13. Decarboxylation: add a sulfuric acid so...
Embodiment 3
[0066] Embodiment 3: a kind of synthetic method of optically active intermediate N-tert-butoxycarbonyl-2-amino-8-nonenoic acid dicyclohexylamine salt, specifically comprises the following steps:
[0067] S1. generate intermediate IV, which comprises the following substeps:
[0068] S11. Alkylation: Add tert-butyl acetamidomalonate and 7-iodo-1-heptene to toluene and water, then add tetrabutylammonium bromide to react under alkaline conditions at 75°C to obtain alkanes Alkylation product; Described alkali is cesium carbonate, sodium hydride and sodium methylate; The molar mass ratio of tert-butyl acetamidomalonate, 7-iodo-1-heptene and tetrabutylammonium bromide is 1:1: 0.4; The molar mass ratio of tert-butyl acetamidomalonate to base is 1:3;
[0069] S12. Hydrolyzing the alkylation product: performing a hydrolysis reaction on the alkylation product obtained in step S11 in an acetic acid solution at 50° C.;
[0070] S13. Decarboxylation: add a phosphoric acid solution with a ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com