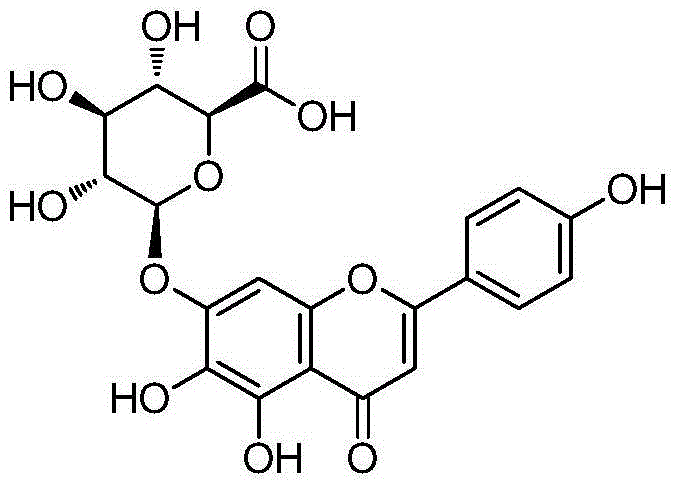
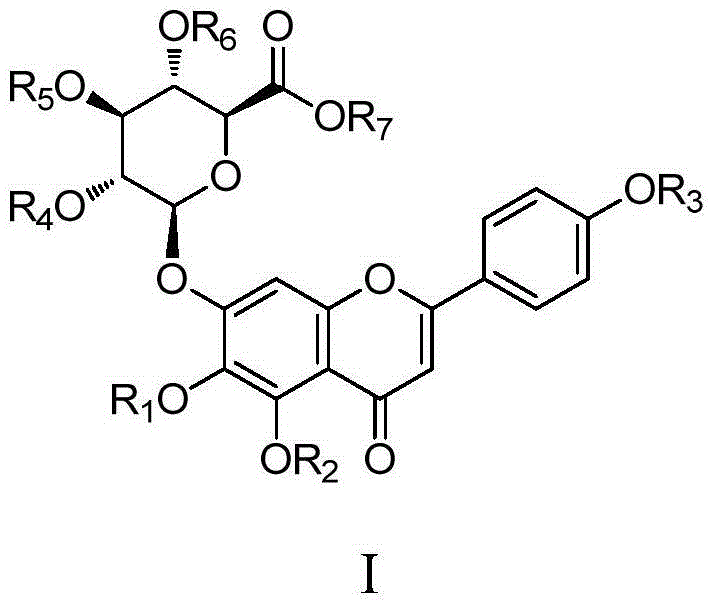
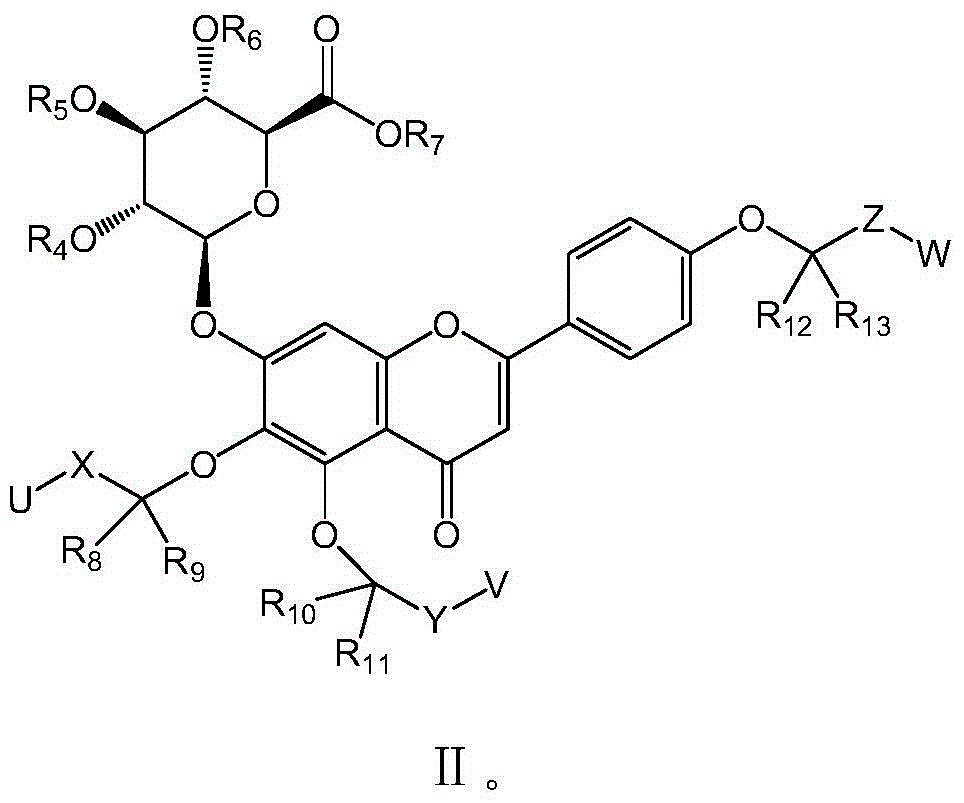
Scutellarin compounds and preparation method and application thereof
A technology of scutellarin and compounds, which is applied in the field of scutellarin compounds and their preparation, can solve the problems of low absolute bioavailability and poor water solubility of scutellarin, and achieve the effect of solving adverse reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] The preparation of embodiment 1 scutellarin sodium phosphate
[0065] Follow the directions below:
[0066]
[0067] (1) Preparation of scutellarin methyl ester of formula 2
[0068] Take 100mL of methanol in a two-necked flask, protect it with nitrogen, cool to 0°C, add acetyl chloride (2mL, 2.8mmol), add scutellarin (Compound 1) (4.62g, 10mmol) in DMF 15mL after 30min, and stir for 15 minutes , slowly return to room temperature, a white solid gradually precipitated out, react at 40°C for 4h, cool at -10°C for 1h, filter, wash the filtrate once with water, and dry the filter cake in vacuum. 3.65 g of yellow solid scutellarin methyl ester (shown in Formula 2) was obtained, with a yield of 77%. Product Characterization Data: 1 HNMR(400MHz,DMSO)δ10.37(s,1H,Ar-OH),8.61(s,1H,Ar-OH),7.94,7.92(d,2H,C 2’,6’ -H),6.99(s,1H,Ar-H),6.95-6.93(d,2H,C 3’,5’ -H),6.81(s,1H,C 8 -H),5.53-5.25(m,5H,Glu-H),4.18(s,3H,OMe).
[0069] (2) the preparation of the whole acetyl scutellari...
Embodiment 2
[0078] The preparation of embodiment 2 scutellarin sodium sulfonate
[0079] Follow the directions below:
[0080]
[0081] (1) preparation of formula 7 compounds
[0082]Place 2.58g of partial acetyl scutellarin of formula 4 and 0.716g of pyridine sulfur trioxide in a dry 50mL single-necked round bottom flask, add 5mL of pyridine and 10mL of DMF under stirring at room temperature, the reaction solution becomes clear, and continue stirring for 24 hours. Evaporate pyridine, add an appropriate amount of water, neutralize the eluent with 2N NaOH solution (2 equivalents of NaOH) to pH 7, separate macroporous resin AB-8, collect by elution with pure water, and evaporate to dryness under reduced pressure to obtain a yellow powder 1.2 g of the solid is the compound of formula 7. Product Characterization Data: MS(ESI):m / z=681.0[M-1] - .
[0083] (2) preparation of scutellarin sodium sulfonate of formula 13'
[0084] Place the compound of formula 7 in a 100mL round bottom flask...
Embodiment 3
[0086] The preparation of embodiment 3 scutellarin sodium methoxyphosphate
[0087] Follow the directions below:
[0088]
[0089] (1) Preparation of formula 9' compound
[0090] Dissolve 1 g of partial acetyl scutellarin of formula 4 in THF, add potassium carbonate, chloromethoxy di-tert-butyl phosphate, after TLC traces the reaction is complete, evaporate THF, add appropriate amount of water, and extract three times with DCM , dichloromethane:methanol ratio of 13:1 was used for column chromatography to obtain 400mg of the compound of formula 9'. Product Characterization Data: MS(ESI):m / z=677.0[M-1] - .
[0091] (2) Preparation of scutellarin methoxyphosphoric acid of formula 10
[0092] 200 mg of the compound of formula 9' was dissolved in 5 mL of 5% TFA in DCM, stirred at room temperature for 1 h, and the solvent was removed by rotary evaporation to obtain 160 mg of scutellarin methoxyphosphate compound (shown in formula 10).
[0093] (3) preparation of scutellarin ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com