Human chorion mesenchymal stem cell isolated culture method
A technology for isolating and culturing stem cells, applied in the field of biomedicine, can solve the problems of limited effect of anti-pollution measures, increase the cost of culture, complicated steps, etc., achieve stable maintenance of cell stemness and cell viability, save enzyme digestion time, and operation process. simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Stripping of human placental chorion
[0050] Before the operation, first wipe the collection container with 75% alcohol for disinfection, then take it into the biological safety cabinet, open the placenta collection container in a sterile environment, and wipe the blood around the mouth of the container with gauze soaked in 75% alcohol to avoid If it drips on the operating table to cause contamination, turn the placenta over with 14cm sterilized tweezers, turn the placenta on the side close to the fetus up, and use 10cm sterilized tweezers to gently peel off the amniotic membrane to expose the chorion layer of the subamniotic membrane. The ophthalmic scissors cut the chorion layer around the umbilical cord along the section of the umbilical cord. During the cutting process, cut along the chorion as much as possible to remove the villi and capillaries connected under the chorion, and place the obtained tissue in 0.9% sodium chloride Wash and remove the blood in the inje...
Embodiment 2
[0052] Primary culture of human chorionic mesenchymal stem cells
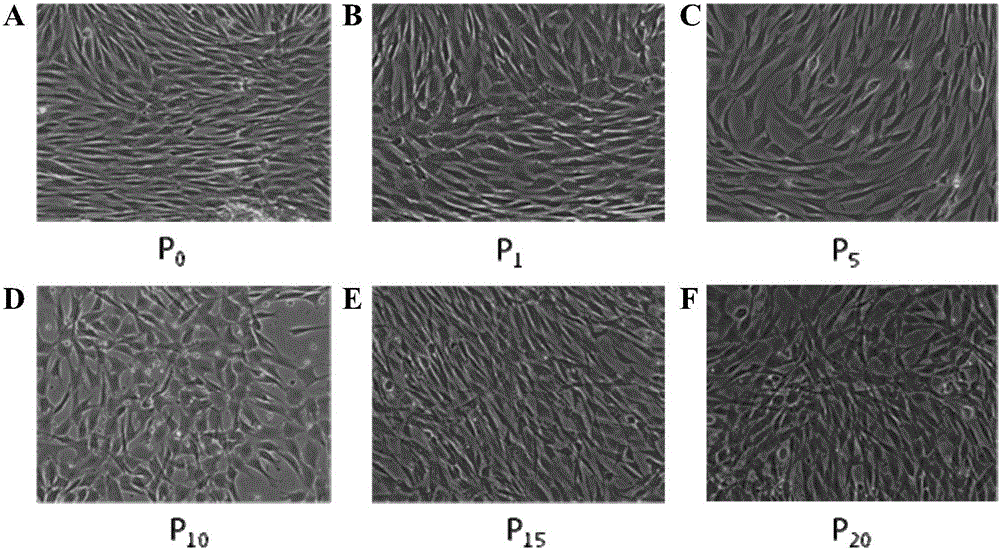
[0053] Put 2ml of detoxified, cleaned, and chopped tissue into a T-75 cell culture bottle, and spread the tissue on the bottom of the cell culture bottle with a Pasteur pipette. Make sure there is a gap between the tissues to prevent the tissue from crawling. A certain space for the cells to grow, the cell culture flask was placed upside down in 5% CO 2 , In a cell culture incubator at 37°C, place it for 30 minutes so that the tissue is firmly attached to the bottom of the cell culture bottle. Slowly add to the part of the culture bottle where there is no tissue adhesion, cover the lid, turn the cell culture bottle over and shake gently, so that the medium is evenly spread between the tissues, and place the cell culture bottle in 5% CO 2 , 37°C cell incubator for static culture, replace the serum-free medium of mesenchymal stem cells after 5 days, observe under the microscope after 7 days of culture, it can be...
Embodiment 3
[0055] Human chorionic mesenchymal stem cells passage
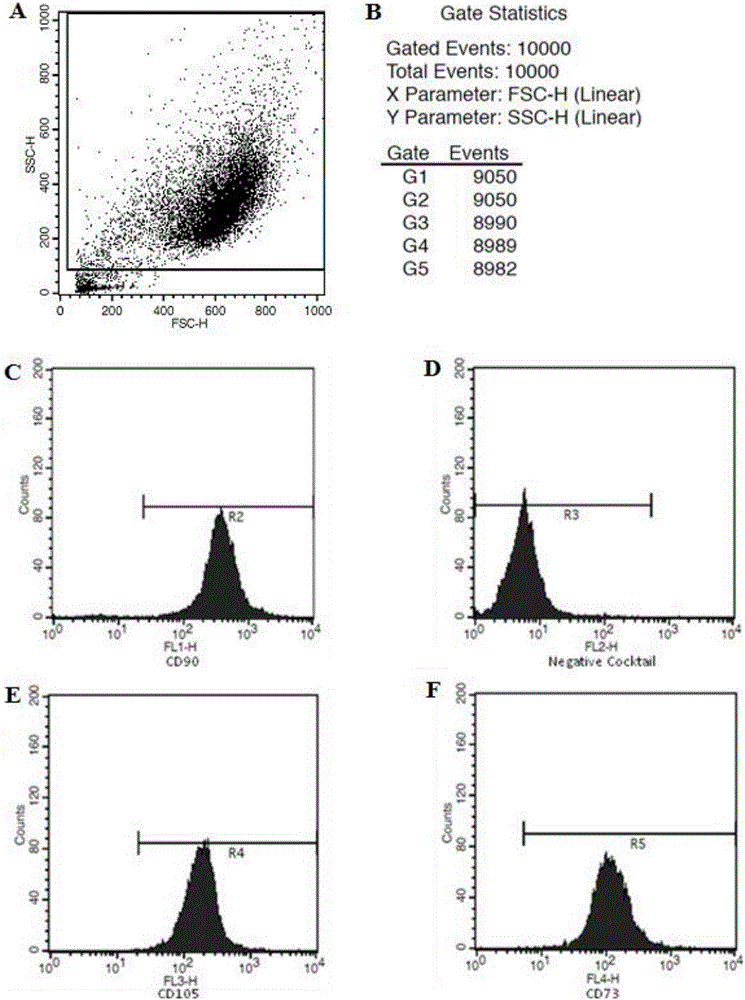
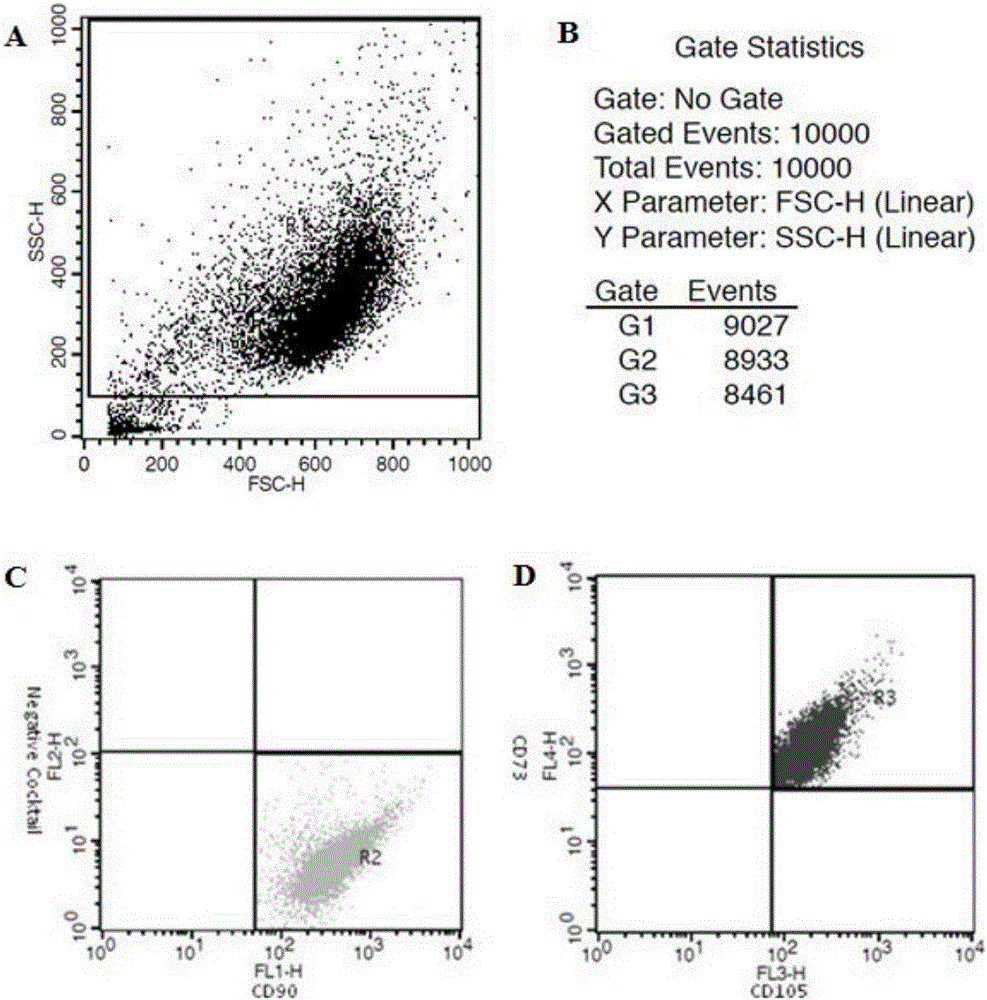
[0056] Remove the medium in the T-75 culture flask completely, add 15ml of PBS to wash the cells once (washing the cells with PBS is not necessary, it can be determined according to the actual situation), remove the PBS, add 3ml of recombinant cell trypsin solution, and put Digest in a 37°C incubator for about 2 minutes. During this process, observe the shrinkage of the adherent cells under an inverted microscope. After all the cells have detached from the bottom of the bottle, pat the side wall of the culture bottle with your palm, and observe under the microscope whether the cells have been digested into individual cells. For cells, add 15ml of mesenchymal stem cell serum-free medium to dilute the recombinant cell trypsin solution to stop the trypsin action, then transfer the cell suspension to a 50ml centrifuge tube, take 200ul of the cell suspension and stain with trypan blue to count the number of viable cells . The...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com