Preparation method for carbon-coated lithium vanadate used as negative electrode material of lithium ion battery
A carbon-coated technology for lithium vanadate and lithium ion batteries, applied in battery electrodes, secondary batteries, circuits, etc., can solve the problems of rapid material capacity decay, increased lithium intercalation voltage, poor electronic conductivity, etc., and achieves improved conductivity. rate, grain size reduction, batch stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
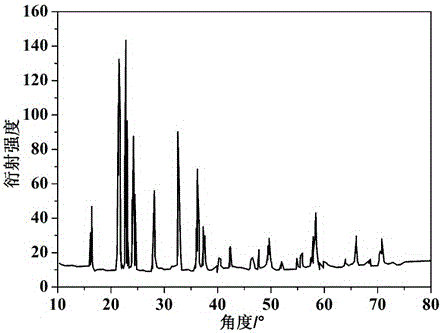
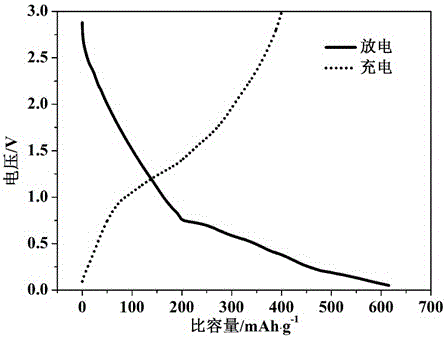
Image
Examples
Embodiment 1
[0032] According to the molar ratio of EDTA: ammonia=1:4, weigh 17.53g of EDTA and add it to 300mL of deionized water, measure 16mL of 25% ammonia water and add it to the EDTA suspension to form a clear and transparent EDTA diamine aqueous solution;
[0033] According to the molar ratio V:Li=1:3, weigh 4.68g ammonium metavanadate and 5.04g lithium hydroxide monohydrate into the EDTA diamine aqueous solution, stir at a temperature of 40℃ to form a yellow-green transparent solution;
[0034] The yellow-green transparent solution was heated to 80°C to evaporate, and after removing the solvent, the blue lithium vanadate precursor was obtained after baking in the air at 120°C for 24 hours;
[0035] Grind the blue lithium vanadate precursor in N 2 / H 2 Pre-fired at 400°C for 4 hours under the protection of mixed gas to obtain a gray lithium vanadate precursor; then grind and crush the gray lithium vanadate precursor and sinter it at 700°C for 8 hours under the protection of high-purity ...
Embodiment 2
[0041] According to the molar ratio of EDTA: ammonia = 1:5, add EDTA to 300 mL of deionized water, and then add ammonia with a mass concentration of 28% to form a clear and transparent EDTA diamine aqueous solution;
[0042] According to the molar ratio V:Li=1:3.5, the V 2 O 5 Add lithium nitrate to the EDTA diamine solution and stir at 30°C to form a yellow-green transparent solution;
[0043] The yellow-green transparent solution is heated to 70°C to evaporate the solvent, and the blue lithium vanadate precursor is obtained after baking in the air at 80°C for 24 hours;
[0044] The blue lithium vanadate precursor was ground and pre-fired at 500°C for 6 hours under the protection of hydrogen to obtain the gray lithium vanadate precursor; the gray lithium vanadate precursor was ground and crushed under the protection of high-purity nitrogen at 900°C After sintering for 6 hours, the carbon-coated lithium vanadate can be obtained after natural cooling.
Embodiment 3
[0046] According to the molar ratio of EDTA: ammonia=1:4.5, weigh 17.53g of EDTA and add it to 300mL of deionized water, measure 18mL of 28% mass fraction of ammonia water and add it to the EDTA suspension to form a clear and transparent EDTA diammonium water solution;
[0047] According to the molar ratio V:Li=1:3, weigh 4.68g of ammonium metavanadate and 12.24g of lithium acetate dihydrate into the EDTA diamine aqueous solution, stir at 50°C to form a yellow-green transparent solution;
[0048] The yellow-green transparent solution is heated to 90°C to evaporate the solvent, and the blue lithium vanadate precursor is obtained after baking in the air at 120°C for 12 hours;
[0049] After grinding the blue lithium vanadate precursor under the protection of high-purity nitrogen, it is calcined at 400℃ for 4h to obtain the gray lithium vanadate precursor; the gray lithium vanadate precursor is ground and crushed in CO 2 Under protection, sintering at 600°C for 12 hours and natural c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com