Three-dimensional porous perovskite catalyst La<x>Sr(1-x)Co<y>Fe<1-y>O<3> and preparation method thereof
A three-dimensional porous, perovskite-type technology, used in fuel cell-type half-cells and secondary battery-type half-cells, structural parts, electrical components, etc. High potential, poor cycle performance, etc., to achieve good electrical conductivity and ionic conductivity, improve capacity and cycle stability, and low cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0032] The preparation method of the three-dimensional porous perovskite catalyst of the present invention is described and illustrated in detail below, and the preparation method specifically includes the following steps:
[0033] 1) La(NO 3 ) 2 ·6H 2 O, Sr(NO 3 ) 2 , Co(NO 3 ) 2 ·6H 2 O, Fe(NO 3 ) 3 9H 2 O The four nitrates are weighed according to the molar ratio x:1-x:y:1-y, then dissolved in a certain amount of methanol and ethylene glycol mixed solution, stirred at a constant temperature of 20-30°C until the nitric acid The salt is all dissolved and mixed well to obtain a nitrate solution. As a preferred embodiment, the volume ratio of methanol to ethanol is 1:1, and the total concentration of the nitrate solution is 1 to 2 mol L -1 .
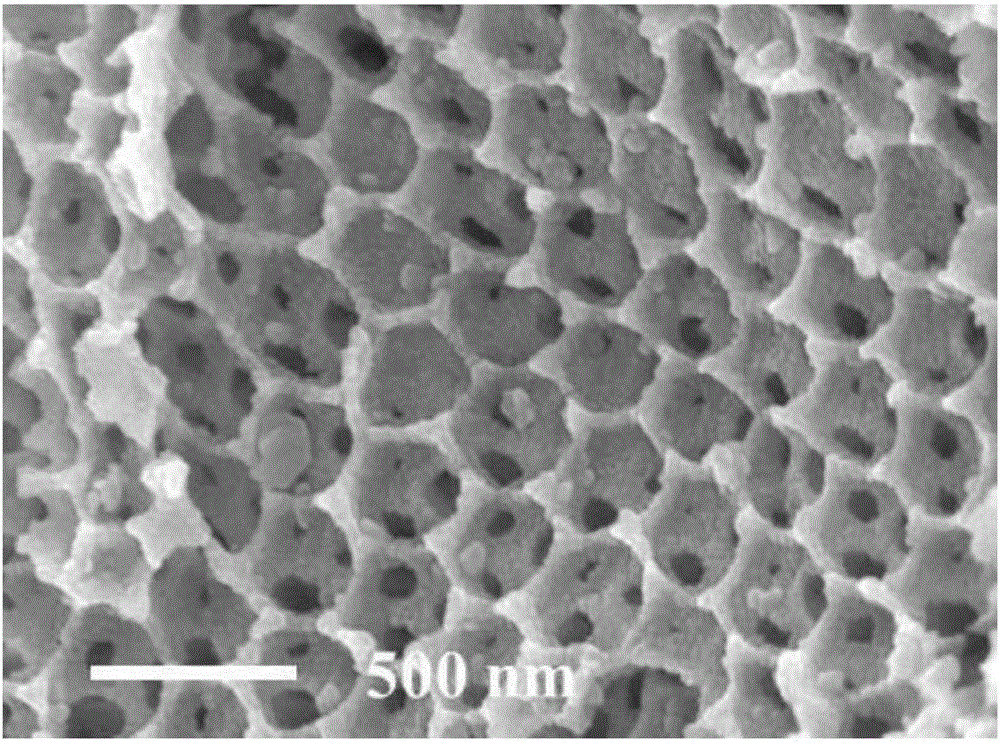
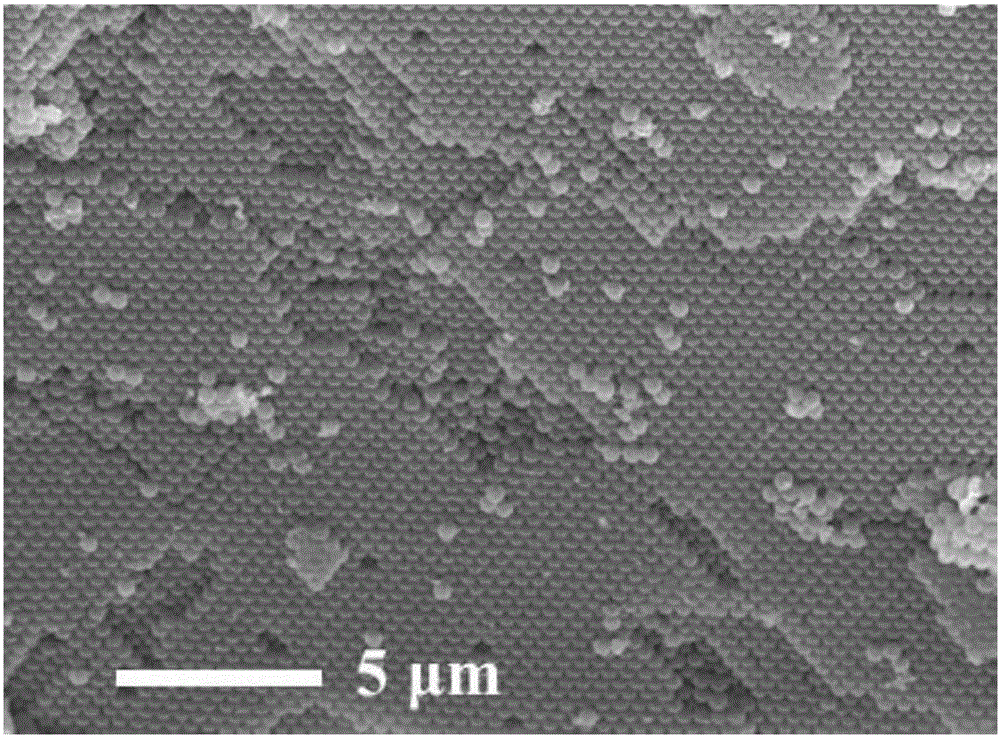
[0034] 2) Soak the PS microsphere (polystyrene microsphere) template in the above nitrate solution, wherein the PS microsphere template is prepared by the microsolution method and then self-assembled by centrifugation. The par...
Embodiment 1
[0042] Prepare PS microsphere template:
[0043] Dissolve 0.065g of sodium dodecylbenzenesulfonate and 0.0489g of potassium persulfate in a mixed solution of 20ml of distilled water and 50ml of ethanol, and place them in a 250ml three-neck flask after dissolving; add about 3ml of styrene to the solution, Access to N 2 The atmosphere was stirred at a constant temperature of 70°C for 6.5 hours; after the stirring was completed, the temperature was lowered, and the mixed solution was transferred to a centrifuge tube for centrifugal washing at a centrifugal rate of 2000r / min for 16 hours, the washing liquid was ethanol, and the number of washings was 3 times; After drying in a freeze-drying oven for 5 hours, orderly arranged polystyrene microsphere templates were obtained.
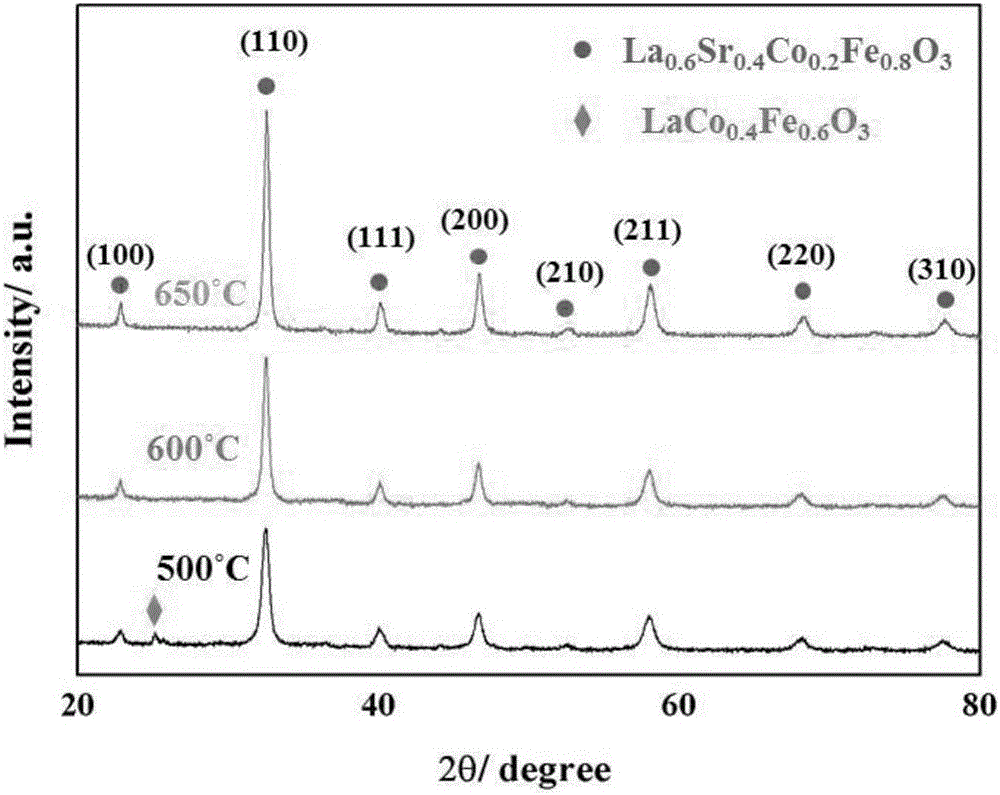
[0044] Preparation of perovskite catalyst La 0.6 Sr 0.4 co 0.2 Fe 0.8 o 3 :
[0045] La(NO 3 ) 2 ·6H 2 O, Sr(NO 3 ) 2 , Co(NO 3 ) 2 ·6H 2 O, Fe(NO 3 ) 3 9H 2 O four kinds of nitrates weighed ...
Embodiment 2
[0047] The LSCF material in Example 1 is used as the cathode catalyst of the non-aqueous lithium-air battery, that is, the LSCF is mixed with the carbon material SuperP and the binder to make a slurry, and the cathode electrode sheet is made on the carbon paper by screen printing , and then assemble the electrode sheet into a battery for testing. In the test, when preparing the cathode slurry, the ratio of the powder to the binder PVDF (polyvinylidenefluoride) was 90:10, and the mass ratio of the catalyst LSCF to the carbon powder SuperP in the powder was 35:55. Specifically include the following steps:
[0048] PVDF is first dissolved in NMP (N-methyl-2-pyrrolidone, N-methyl-2-pyrrolidone) solvent, and then the catalyst LSCF and carbon powder SuperP powder are weighed according to the stated ratio, and the powder and binder PVDF are placed on the agate Grind in a mortar, add an appropriate amount of NMP dropwise, and grind for about 2.5 hours to obtain a uniform slurry; use ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com