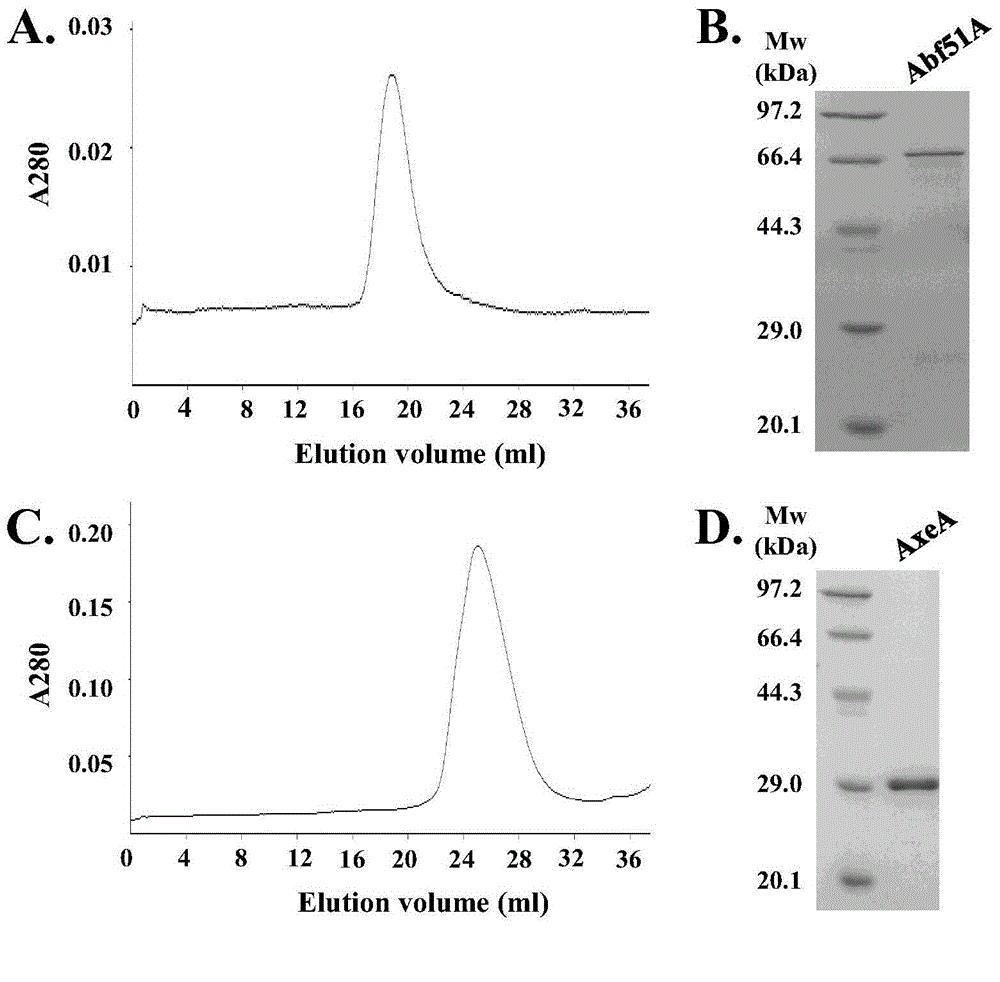
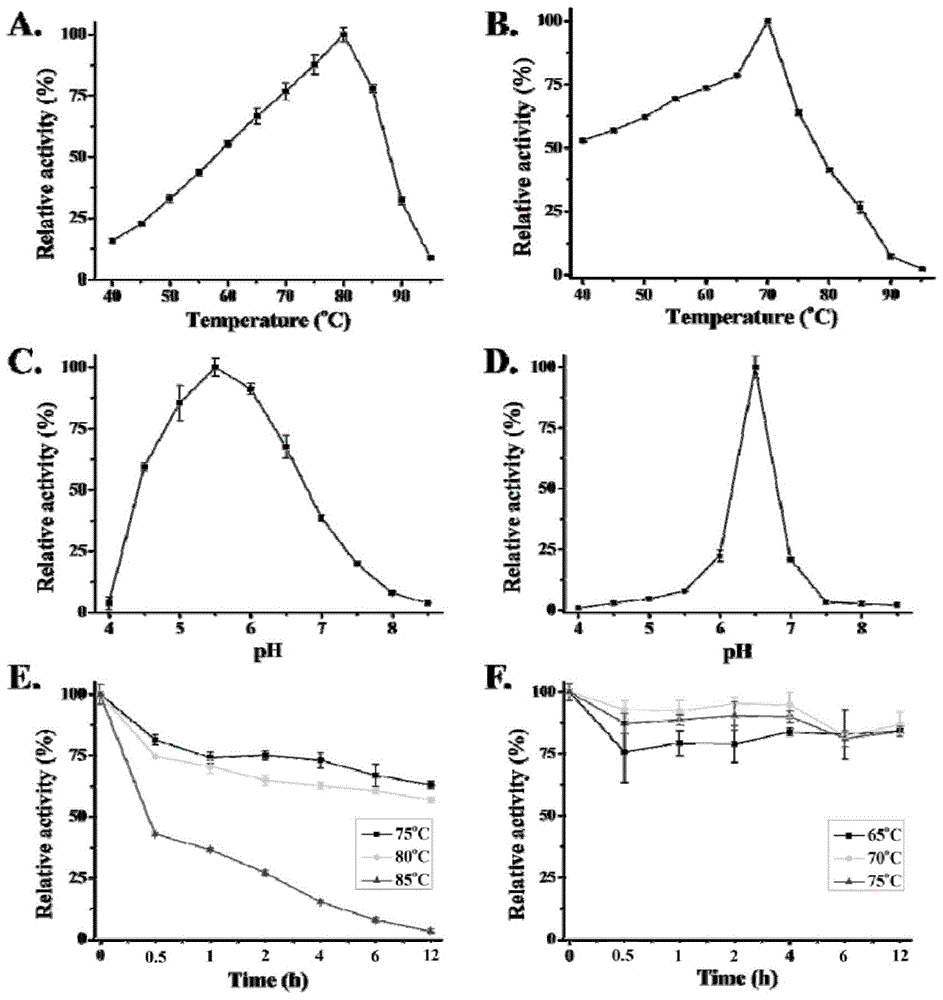
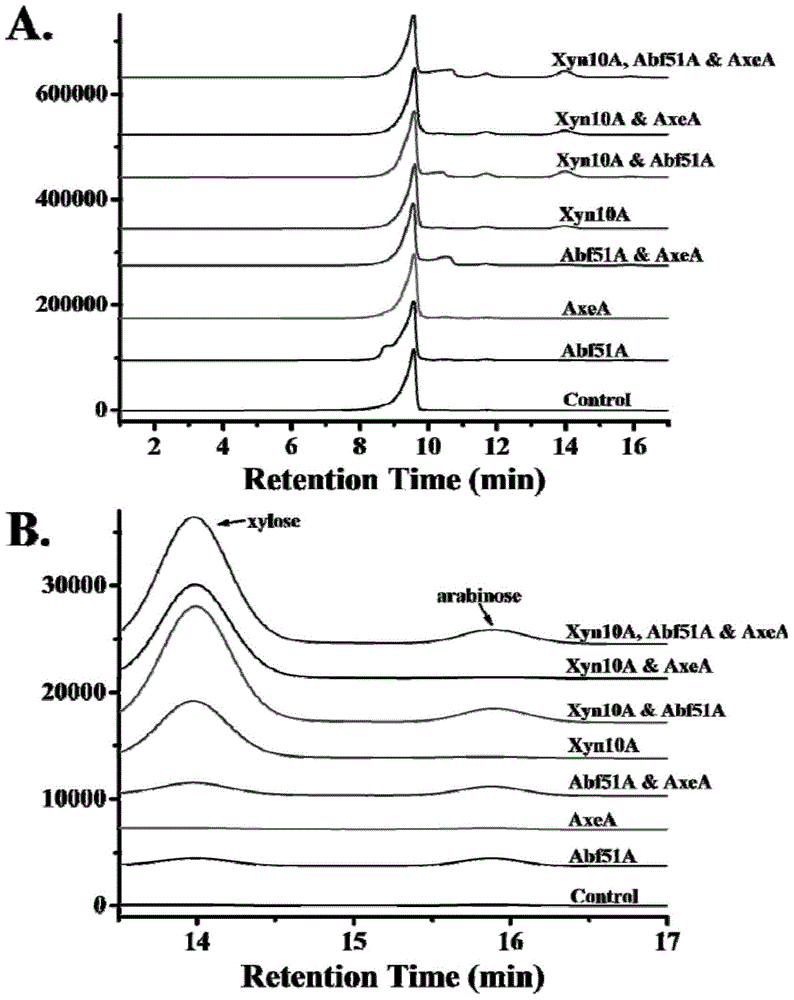
A high-temperature alpha-L-arabinfuranosidease gene, a high-temperature acetylxylan esterase gene, and protein expression and applications of the genes
An acetyl xylan ester and furanosidase technology, which is applied in the fields of genetic engineering and biomass utilization, can solve the problems of being unsuitable for industrialized large-scale production, complex components of xylan degrading enzymes, harsh growth conditions, etc. Improved biodegradability, high optimum reaction temperature and good thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1: Construction of high-temperature α-L-arabinofuranosidase gene abf51A and acetylxylan esterase gene axeA engineering bacteria
[0036] 1.1 Genomic DNA extraction
[0037] The bacterial genome of C. lactoaceticus6A was extracted with a bacterial genomic DNA extraction kit to obtain genomic DNA, which was frozen at -20°C for future use.
[0038] 1.2 Primer design
[0039] According to the published genome information of C.lactoaceticus6A, the α-L-arabinofuranosidase and acetylxylan esterase genes were predicted, and the following 2 pairs of primers were designed:
[0040] The primers used to amplify the α-L-arabinofuranosidase gene are as follows:
[0041] abf51A-F5'-GCCGCGCGGCAGCATGAAAAAAGCAAAAGTCATCTAC-3'
[0042] abf51A-R5'-GCGGCCGCAAGCGTTTAATTTTCTTCTTCTTTAACCTG-3'
[0043] The primers used to amplify the acetyl xylan esterase gene are as follows:
[0044] axeA-F5'-GCCGCGCGGCAGCATGATACCACTTTGGGAAAATC-3'
[0045] axeA-R5'-GCGGCCGCAAGCGTTTAAAACATTATATCCTA...
Embodiment 2
[0056] Example 2: Expression of recombinant α-L-arabinofuranosidase Abf51A and acetylxylan esterase AxeA in Escherichia coli
[0057] The recombinant bacteria E. coli Rosetta (DE3) were inoculated in 5 ml of LB liquid medium containing 50 μg / ml kanamycin according to the inoculum amount of 1%, and cultured overnight at 37° C. with shaking at 200 rpm. Transfer to 200ml LB liquid medium containing 50μg / ml kanamycin according to the same inoculum amount, shake culture at 200rpm at 37°C until OD 600 When it reaches about 0.4-0.6, add IPTG to a final concentration of 0.1 mM, and continue shaking culture at 200 rpm at 37°C for 4-6 hours. After the cultivation, the cells were collected by centrifugation at 4000 g for 15 min, and resuspended in 30 ml BindingBuffer (50 mM Tris-HCl pH 7.5, 300 mM NaCl) to collect the cells. After sonication, the supernatant obtained by centrifugation at 10,000g at 4°C for 15 minutes is the crude enzyme solution.
Embodiment 3
[0058] Example 3: Expression of recombinant α-L-arabinofuranosidase Abf51A and acetylxylan esterase AxeA in Trichoderma reesei
[0059] Using the C. lactoaceticus6A genome as a template, design primers to amplify to obtain α-L-arabinofuranosidase gene abf51A and acetylxylan esterase gene axeA, respectively, and connect them into vector pSKCST to obtain expression vectors pSKCST-abf51A and pSKCST-axeA respectively . The expression vectors pSKCST-abf51A and pSKCST-axeA were linearized and then transformed into T.reesei protoplasts. After the transformants grew out, the genomic DNA of the transformants was extracted, and the integration of exogenous genes in the transformants was identified by PCR. Use a cutter to cut the colonies of transformants on the selective plate into 0.3 cm diameter bacterial blocks, take 5 blocks from each transformant, transfer them to the EG screening plate, and culture them at 28°C for 2-3 days. Regularly observe and record the growth diameter. Sele...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com