Preparation method and application of double-photon fluorine ion fluorescent probe compound
A technique for fluorescent probes and compounds, applied in the field of fluorescent probes, can solve the problems of inability to quantitatively measure fluoride ion content, the probe does not report a working curve, and autofluorescence will interfere, achieving good sensitivity and selectivity, and good promotion. Prospect, good water solubility effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Embodiment 1, the synthesis of probe molecule compound I
[0038] (a) Take 1.106g of 2,4-dihydroxybenzaldehyde and 0.816g of imidazole in a three-necked flask under nitrogen protection. Take 1.3mL of cyclohexenone and add it into the flask; add 6mL of tetrahydrofuran and 6mL of water into the flask. React at ℃ for 72h, then add 20mL of 1M dilute hydrochloric acid, extract the aqueous phase with ethyl acetate, evaporate the solvent, use the mixture of ethyl acetate and n-hexane with a volume ratio of 1:6 as the developing solvent, and separate by column chromatography to obtain 0.46 g compound II, yield 19%;
[0039] (b) Dissolve 0.1g of compound II, 0.331g of tert-butyldimethylchlorosilane, and 0.163g of imidazole in 10mL of dichloromethane, react for 8 hours at room temperature under nitrogen atmosphere, distill off the solvent, and use a volume ratio of 10:1 A mixed solvent of petroleum ether and ethyl acetate was used as a developer and separated by column chromatog...
Embodiment 2
[0040] Embodiment 2, fluorescence experiment
[0041] Take the fluorescent probe compound prepared in Example 1, dissolve it in an aqueous solution containing 5% ethanol, adjust the pH=7.4 with HEPES buffer solution; obtain the fluorescent probe solution, and set aside;
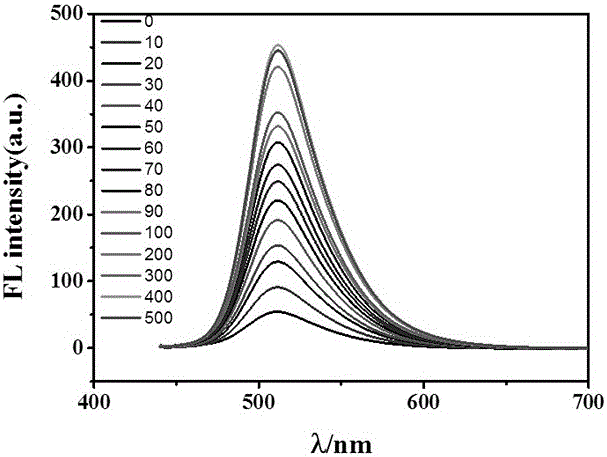
[0042] 1. Take the fluorescent probe solution and divide it into 11 groups, 10mL in each group. Among them, 1 group does not add anion, and 10 groups add CO 3 2? , SO 4 2? , NO 3 ? , Cl ? , I ? 、Br ? 、CH 3 COO ? , N 3 ? 、PO 4 3? , F ?solution, so that the concentration of the probe compound contained in each group of solutions is 10 μM, and the anion concentration is 300 μM, so that the molar ratio of the anion to the probe compound is 30:1; the excitation wavelength is 422 nm, and the fluorescence intensity is tested by a fluorescence photometer. The invention probe solution itself has no fluorescence. Once fluoride ions are added, the fluorescence of the solution at 511nm increases rapidly, b...
Embodiment 3
[0044] Embodiment 3, cell imaging experiment
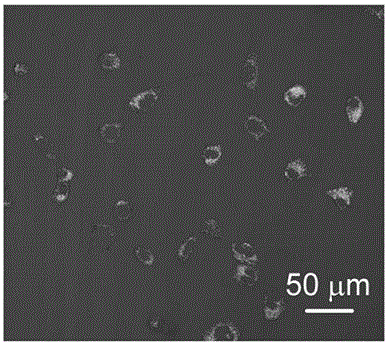
[0045] MCF-7 cells were cultured in 1 mL cell culture medium containing 10% bovine fetal serum for 12 hours, then treated with 100 μM fluoride ion for 10 minutes, and then treated with 10 μM fluorescent probe of the present invention for 30 minutes. Cells were excited with a light source with an excitation wavelength of 534 nm and imaged under a confocal microscope. Such as image 3 A picture of MCF-7 cells cultured with the addition of fluoride ions for the fluorescent probe of the present invention.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com