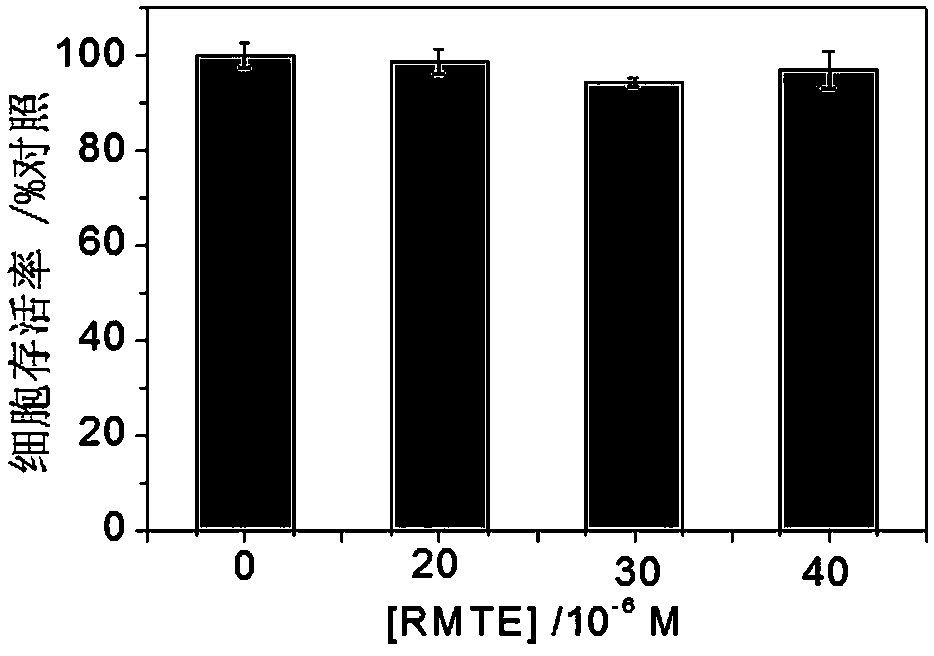
Compounds based on rhodamine b and aminoethyl sulfide, methods for their preparation and applications
A technology of aminoethyl sulfide and compounds, applied to the application of mercury ion probes in the detection of mercury ions, based on the field of rhodamine B and aminoethyl sulfide compounds and their preparation, can solve the problem of irreversible mercury ion reactions, Problems such as rare raw materials and difficult synthesis can achieve the effects of industrial application, mild reaction conditions and good application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment one: Hg 2+ Preparation of probe RMTE
[0034] According to the ratio of raw materials in Table 1 (molar ratio of rhodamine B and aminoethyl sulfide) and reaction conditions, with dichloromethane as solvent, N,N-diisopropylethylamine as additive, in N 2 Under protection, stir the reaction, cool to room temperature, remove the solvent by rotary evaporation, and separate by column chromatography, the eluent is methanol / chloroform / petroleum ether, 1 / 12 / 2 (v / v / v), dried to obtain Target product RMTE as yellow solid powder.
[0035] NMR, 1 H NMR (400 MHz, CDCl 3 ), δ / ppm: 1.16 ( t , 12H, J=6.8 Hz), 2.23( t , 2H, J=8 Hz), 2.50 ( t , 2H, J=6.4 Hz), 2.77 ( t , 2H, J=6.4 Hz), 3.27–2.37( m , 10H), 3.66 (s, 2H), 6.27 ( d , 2H, J=6.4 Hz), 6.37 ( d , 2H, J=2.4 Hz), 6.44( s , 2H), 7.08–7.10 ( m , 1H), 7.43–7.46 ( m , 2H), 7.89–7.91 ( m , 1H), 13 C NMR (300MHz, CDCl 3 ), Δ / PPM: 12.35, 29.37, 34.71, 40.24, 40.83, 44.31, 64.69, 97.34,105.62, 107.96, 122.63, 1...
Embodiment 2
[0038] Embodiment two: RMTE to Hg 2+ the response to
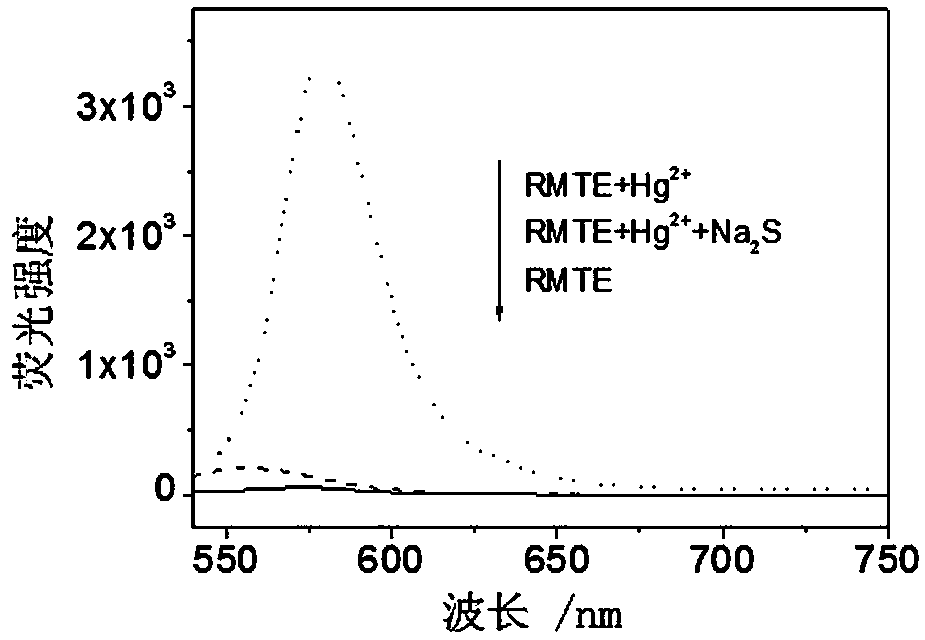
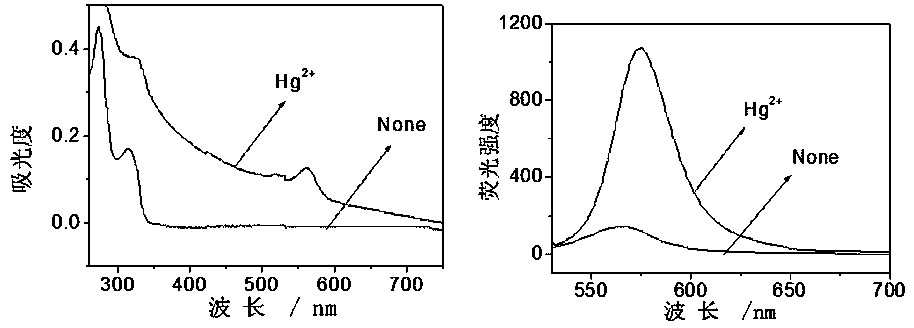
[0039] CH at RMTE 3 Add Hg to CN / HEPES buffer solution (1 / 1, v / v, pH=7.05) 2+ , measure the UV-Vis absorption spectrum and fluorescence spectrum before and after adding ions, the results are as follows figure 1 , solvent: CH 3 CN / HEPES buffer solution (1 / 1, v / v, pH=7.05), concentration: 20 μM (RMTE), 200 μM (Hg 2+ ). Excitation wavelength: 520 nm, slit width: 5 nm. Hg 2+ The addition of the RMTE UV-Vis absorption spectrum at 560 nm appears a new absorption peak ( figure 1 Left panel), the fluorescence was significantly enhanced by 33.9 times ( figure 1 right).
[0040] CH at RMTE 3 Add Hg to CN / HEPES buffer solution (2 / 8, v / v, pH=7.05) 2+ , measure the UV-Vis absorption spectrum and fluorescence spectrum before and after adding ions, the results are as follows figure 2 , solvent: CH 3 CN / HEPES buffer solution (2 / 8, v / v, pH=7.05), concentration: 20 μM (RMTE), 200 μM (Hg 2+ ). Excitation wavelength: 520 nm, s...
Embodiment 3
[0043] Embodiment three: RMTE and Hg 2+ concentration relationship
[0044] Figure 4 for containing different concentrations of Hg 2+ UV-Vis absorption spectrum (left) and fluorescence spectrum (right) of RMTE solution, solvent: CH 3 CN / HEPES buffer solution (1 / 99, v / v, pH=7.05), concentration: 20 μM (RMTE), Hg 2+ Concentrations from bottom to top are 0, 10, 20, 30, 40, 60, 80, 100, 120, 140, 160, 180 and 200 μM, insets are RMTE absorbance (A) and Hg at 561 nm, respectively 2+ The relationship between concentration and RMTE fluorescence intensity (F) at 578 nm vs. Hg 2+ The relationship between concentration, excitation wavelength: 520 nm, slit width: 5 nm. from Figure 4 As can be seen from the left figure, with Hg 2+ As the concentration increases, the absorbance of RMTE at 561 nm first increases and then tends to be stable. in Hg 2+ In a wide range of concentrations, the absorbance A and Hg 2+ The concentration has a good linear relationship, and the linear equat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com