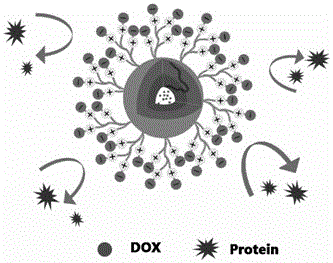
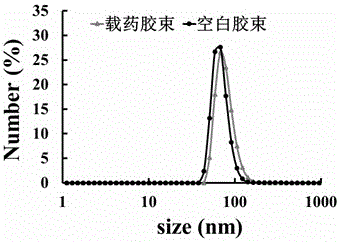
Zwitter-ion-contained multiple acid-sensitive anti-tumor drug-loading micelle and preparation method and application thereof
A zwitterion, acid-sensitive technology, applied in the field of multiple acid-sensitive anti-tumor drug-loaded micelles and their preparation, can solve the problems of difficult to achieve drug treatment concentration, the inability of nanoparticles to respond quickly, etc., and achieve low toxic side effects and high sensitive response. Sexual and anti-tumor efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
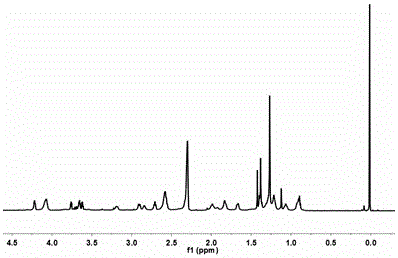
[0050] Example 1: Preparation of 2,2-bis(acryloyloxy-1-ethoxy)propane
[0051] First, mix hydroxyethyl acrylate and 2,2-dimethoxypropane in a molar ratio of 2:1, add benzene and p-toluenesulfonic acid, and distill the benzene-methanol azeotrope under the reaction conditions of 58°C out to obtain the initial product. The obtained primary product was purified by silica gel column chromatography, eluted with a mixed solvent of n-hexane and dichloroethane (1:1), and the eluent was removed by rotary evaporation to obtain a colorless oily liquid. After the product ADA.
Embodiment 2
[0052] Example 2: Preparation of Carboxybetaine Methacrylate (CBMA)
[0053] Dissolve 0.11 mmol of 2-(dimethylamino)ethyl methacrylate in 50 mL of anhydrous acetone under vigorous stirring, and slowly dissolve 0.14 mmol of β-propiolactone in 10 mL of anhydrous acetone under nitrogen protection Add dropwise to the above solution. Under the protection of nitrogen, the reaction was carried out at 4° C. for 5 h to obtain a white powder. Afterwards, the obtained white powder was washed successively with anhydrous acetone and anhydrous ether, and dried in a vacuum oven to obtain a white powder product.
Embodiment 3
[0054] Example 3: Preparation of polycarboxybetaine methacrylate (pCBMA)
[0055] Use ATRP method (atom transfer radical polymerization) reaction to prepare polycarboxylate betaine methacrylate, use the product synthesized in Example 2 as a reaction monomer, and use ethyl 2-bromoisobutyrate as an initiator , with 1,1,4,7,10,10-hexamethyltriethylenetetramine as a ligand, put into a dry branch bottle at a molar ratio of monomer:initiator:ligand=16.6:1:2 In the process, three vacuum-nitrogen cycles were performed to ensure the oxygen-free environment of the reaction system. Methanol:N,N-dimethylformamide (1:1) was injected into the reaction system with a syringe as a solvent, and cuprous bromide was added after three vacuum-nitrogen cycles. The reaction system was sealed and reacted at 60 °C for 24 h. The reaction system was communicated with air to terminate the reaction. The initial reaction product obtained is dialyzed in neutral water with a 500-1000 molecular weight cut-o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com